Elements In A Periodic Group Have Similar
News Leon
Apr 07, 2025 · 6 min read
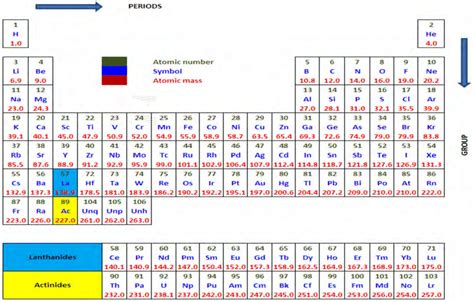
Table of Contents
Elements in a Periodic Group Have Similar: A Deep Dive into Group Properties and Periodic Trends
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and recurring properties. One of the most fundamental observations is that elements within the same group (vertical column) exhibit strikingly similar chemical and physical characteristics. This similarity stems from their shared number of valence electrons, the electrons residing in the outermost shell and playing a crucial role in chemical bonding. This article delves deep into the reasons behind this similarity, exploring the periodic trends that govern group properties and illustrating these concepts with specific examples.
Understanding Valence Electrons: The Key to Group Similarity
The number of valence electrons is the defining factor that determines the chemical behavior of an element. Elements in the same group possess the same number of valence electrons, leading to similar electron configurations in their outermost shell. This similarity in electronic structure dictates how these elements interact with other atoms, resulting in comparable chemical reactivity and bonding patterns.
Group 1: Alkali Metals – A Case Study in Similarity
Let's examine Group 1, the alkali metals (lithium, sodium, potassium, rubidium, cesium, and francium). All alkali metals have one valence electron. This single electron is easily lost, resulting in the formation of +1 ions. This shared characteristic explains their similar properties:
-
High Reactivity: Their tendency to lose their single valence electron makes them highly reactive with water, oxygen, and halogens. Reactions with water, for instance, produce hydrogen gas and a metal hydroxide. The vigor of this reaction increases as you move down the group (from lithium to francium).
-
Low Ionization Energies: The energy required to remove the single valence electron is relatively low, further contributing to their reactivity.
-
Low Electronegativity: They have a low tendency to attract electrons, favoring the loss of their valence electron.
-
Metallic Character: All alkali metals are soft, silvery-white metals with low melting and boiling points.
The similarities within Group 1 are not merely coincidental. They are a direct consequence of their identical valence electron configuration, illustrating the fundamental principle governing group properties.
Group 17: Halogens – Another Example of Group Trends
Group 17, the halogens (fluorine, chlorine, bromine, iodine, and astatine), presents another compelling case study. These elements have seven valence electrons, one electron short of a stable octet. This characteristic drives their chemical behavior:
-
High Reactivity: Their strong tendency to gain one electron to achieve a stable octet makes them highly reactive. They readily form -1 ions through ionic bonding.
-
High Electronegativity: Halogens possess high electronegativity, reflecting their strong attraction for electrons.
-
Varied Physical States: The halogens exhibit a progression in physical states: fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid. This trend is attributed to the increasing strength of intermolecular forces as the atomic size and mass increase down the group.
-
Formation of Diatomic Molecules: Halogens exist as diatomic molecules (F₂, Cl₂, Br₂, I₂) due to the covalent bonding between two halogen atoms, each sharing an electron to achieve a stable octet.
Periodic Trends and Their Influence on Group Properties
While elements within a group share many similarities, gradual changes – known as periodic trends – occur as you move down the group. These trends are largely influenced by:
-
Increasing Atomic Radius: As you progress down a group, the atomic radius (the distance from the nucleus to the outermost electron) increases. This is because additional electron shells are added, increasing the distance between the nucleus and the valence electrons. This trend impacts various properties, including reactivity and ionization energy.
-
Decreasing Ionization Energy: The ionization energy, the energy required to remove an electron, decreases down a group. The increased distance between the nucleus and the valence electrons weakens the electrostatic attraction, making it easier to remove an electron.
-
Decreasing Electronegativity: Electronegativity, the ability of an atom to attract electrons in a chemical bond, generally decreases down a group. The increasing distance between the nucleus and the valence electrons reduces the nucleus's pull on shared electrons.
-
Increasing Metallic Character: In general, metallic character increases down a group. This is primarily because the valence electrons are further from the nucleus and are more easily lost, leading to the characteristic properties of metals (e.g., conductivity, malleability).
Exceptions and Nuances
While the general trends are consistent, exceptions and nuances exist. For instance, the reactivity of halogens is not uniformly predictable. While fluorine is the most reactive halogen, some anomalies in reactivity are observed due to factors like bond strength and electron-electron repulsion. Similarly, the transition metals show more complex trends compared to the main group elements due to the involvement of d-electrons in bonding.
Beyond Chemical Properties: Physical Properties and Group Trends
The similarities between elements in a group extend beyond chemical properties. Physical properties also exhibit patterns, though these trends are often less pronounced than chemical ones. For example:
-
Melting and Boiling Points: Melting and boiling points often show a trend within a group, generally increasing with increasing atomic mass due to the stronger interatomic or intermolecular forces. However, this trend isn't always straightforward and depends on the type of bonding and the nature of intermolecular interactions.
-
Density: Density often increases down a group due to the increase in atomic mass. However, the increase in atomic size can counteract this effect, leading to variations in the density trend depending on the group.
-
Conductivity: The electrical and thermal conductivity of metals usually increases down a group due to the increased availability of delocalized electrons.
These physical property trends, while less predictable than chemical trends, reinforce the underlying connection between atomic structure and observed properties.
Applications and Significance of Group Properties
Understanding the similarities and trends within groups is crucial in various fields:
-
Predicting Chemical Reactivity: Knowing the group properties allows chemists to predict the reactivity of elements and design appropriate reactions. This is fundamental in synthesis, industrial processes, and materials science.
-
Developing New Materials: The understanding of how element properties change within a group helps in designing new materials with specific properties. For example, the knowledge of alkali metal properties has led to applications in batteries and other energy storage technologies.
-
Environmental Chemistry: Knowing the environmental behavior of elements within a group helps in understanding their impact on the environment and in developing remediation strategies.
Conclusion: The Power of the Periodic Table
The periodic table is not merely a chart; it is a powerful tool reflecting the fundamental principles of atomic structure and their influence on the properties of elements. The remarkable similarities exhibited by elements within the same group underscore the critical role of valence electrons in determining chemical and physical behavior. Understanding these periodic trends and group properties is fundamental to advancing our knowledge of chemistry and its various applications. Further research continues to refine our understanding of these trends, revealing further nuances and exceptions that enrich our understanding of the chemical world. The consistent observation that elements in a periodic group share similar characteristics remains a testament to the power and elegance of the periodic table.
Latest Posts
Latest Posts
-
A Rectangle Is Also A Parallelogram
Apr 08, 2025
-
Algal Cell Wall Is Made Up Of
Apr 08, 2025
-
A Hydrogen Atom Is In The Ground State It Absorbs
Apr 08, 2025
-
How Many Nm In 1 Cm
Apr 08, 2025
-
The Basic Structural Unit Of Compact Bone Is The
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about Elements In A Periodic Group Have Similar . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.