Effective Nuclear Charge Zeff Is Defined As
News Leon
Mar 15, 2025 · 6 min read
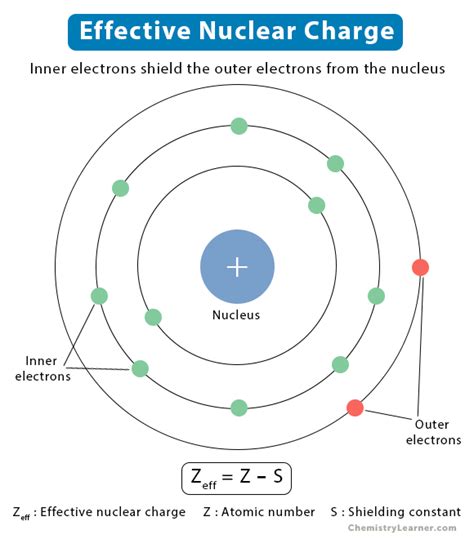
Table of Contents
Effective Nuclear Charge (Z<sub>eff</sub>): A Deep Dive into Atomic Structure and its Implications
Effective nuclear charge (Z<sub>eff</sub>), a cornerstone concept in chemistry and atomic physics, significantly impacts an atom's properties and behavior. Understanding Z<sub>eff</sub> is crucial for predicting various chemical and physical phenomena, including atomic size, ionization energy, and electron affinity. This comprehensive article delves into the definition, calculation, trends, and applications of effective nuclear charge.
What is Effective Nuclear Charge (Z<sub>eff</sub>)?
The effective nuclear charge is the net positive charge experienced by an electron in a multi-electron atom. It's not the full positive charge of the nucleus (Z, the atomic number) because the negatively charged electrons in inner shells shield or screen the outer electrons from the full attractive force of the nucleus. This shielding effect reduces the electrostatic attraction between the nucleus and the outer electrons. Therefore, Z<sub>eff</sub> is always less than Z.
The simple equation representing this relationship is:
Z<sub>eff</sub> = Z - S
Where:
- Z is the atomic number (number of protons in the nucleus)
- S is the screening constant (representing the shielding effect of inner electrons)
The screening constant (S) is not a straightforward value. Its calculation depends on the complex interactions between electrons and involves considerations of orbital penetration and electron-electron repulsion. Various models exist to estimate S, including Slater's rules, which provide a relatively simple and effective method.
Understanding Shielding and Penetration
Shielding occurs when inner electrons partially block the attractive force of the nucleus on outer electrons. Electrons in the same shell (principal quantum number, n) and subshell (angular momentum quantum number, l) shield each other less effectively than electrons in inner shells. This is due to differences in electron density and orbital shapes.
Penetration refers to the ability of an electron in a particular subshell to approach the nucleus closely. Electrons in s orbitals penetrate more effectively than those in p orbitals, which in turn penetrate more effectively than d or f orbitals. This increased penetration leads to less shielding and a higher effective nuclear charge experienced by those electrons. This explains why, for example, an electron in a 2s orbital experiences a higher Z<sub>eff</sub> than an electron in a 2p orbital within the same shell.
Calculating Effective Nuclear Charge: Slater's Rules
One common approach to estimating the screening constant (S) and therefore Z<sub>eff</sub> is Slater's rules. These rules provide a simplified way to approximate the shielding effect based on the electron configuration of the atom.
Slater's Rules:
-
Write the electron configuration of the atom in the following order: (1s)(2s,2p)(3s,3p)(3d)(4s,4p)(4d)(4f) etc.
-
Group the electrons into shells: Electrons in the same group contribute differently to the shielding constant.
-
Calculate the shielding constant (S) for each electron:
-
Electrons in the same group (n, l): Each electron contributes 0.35 (except for the 1s electrons, where each electron contributes 0.30).
-
Electrons in the inner shells: Each electron in the shell immediately preceding the electron under consideration contributes 0.85. Each electron in the shells two or more levels below contributes 1.00.
-
For d and f electrons, electrons in the same n value, contribute 0.35. Electrons in shells with a lower n value contribute 1.00
-
-
Calculate the effective nuclear charge (Z<sub>eff</sub>): Subtract the shielding constant (S) from the atomic number (Z): Z<sub>eff</sub> = Z - S
Example: Let's calculate the Z<sub>eff</sub> for a 3p electron in chlorine (Cl, Z=17). The electron configuration of Cl is 1s²2s²2p⁶3s²3p⁵.
-
The electron configuration is already in the correct order.
-
The groups are: (1s), (2s,2p), (3s,3p).
-
For the 3p electron:
- Shielding from other 3p electrons: 0.35 x 5 = 1.75
- Shielding from 3s electrons: 0.85 x 2 = 1.70
- Shielding from 2s and 2p electrons: 1.00 x 8 = 8.00
- Shielding from 1s electrons: 1.00 x 2 = 2.00
- Total shielding constant (S) = 1.75 + 1.70 + 8.00 + 2.00 = 13.45
-
Z<sub>eff</sub> = Z - S = 17 - 13.45 = 3.55
Therefore, a 3p electron in chlorine experiences an effective nuclear charge of approximately 3.55.
Periodic Trends in Effective Nuclear Charge
Z<sub>eff</sub> exhibits predictable trends across the periodic table, influencing various atomic properties:
-
Across a period (left to right): Z<sub>eff</sub> generally increases. While the number of shielding electrons increases, the increase in nuclear charge (Z) outweighs the increase in shielding. The added protons exert a stronger pull on the outer electrons, leading to a higher effective nuclear charge.
-
Down a group (top to bottom): Z<sub>eff</sub> increases slightly or remains relatively constant. The increase in shielding with added shells roughly cancels the increase in nuclear charge.
Implications of Effective Nuclear Charge
The effective nuclear charge plays a crucial role in determining several key atomic and molecular properties:
1. Atomic Size:
A higher Z<sub>eff</sub> results in a stronger attraction between the nucleus and the valence electrons, leading to a smaller atomic radius. Across a period, atomic size decreases due to the increasing Z<sub>eff</sub>. Down a group, the atomic size increases despite the slightly increasing Z<sub>eff</sub>, primarily due to the addition of electron shells.
2. Ionization Energy:
Ionization energy is the energy required to remove an electron from an atom. A higher Z<sub>eff</sub> leads to a higher ionization energy because the valence electrons are more tightly bound to the nucleus. This is consistent with the trends observed across periods and down groups.
3. Electron Affinity:
Electron affinity is the energy change associated with adding an electron to a neutral atom. A higher Z<sub>eff</sub> generally leads to a higher electron affinity because the atom more readily accepts an electron to achieve a more stable electron configuration.
4. Electronegativity:
Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond. A higher Z<sub>eff</sub> implies a higher electronegativity, indicating a stronger tendency for the atom to pull electrons towards itself in a bond.
5. Chemical Reactivity:
Z<sub>eff</sub> influences the chemical reactivity of an element. Elements with high Z<sub>eff</sub> tend to be more reactive, as their valence electrons are more readily involved in chemical reactions.
Advanced Considerations and Refinements
While Slater's rules provide a useful approximation, they are a simplified model. More sophisticated methods, such as Hartree-Fock calculations and Density Functional Theory (DFT), offer more accurate estimations of Z<sub>eff</sub>. These methods account for electron correlation and electron-electron repulsion more comprehensively.
Furthermore, the concept of Z<sub>eff</sub> is not limited to neutral atoms. It can also be applied to ions, where the effective nuclear charge experienced by the remaining electrons changes due to the loss or gain of electrons.
Conclusion
Effective nuclear charge (Z<sub>eff</sub>) is a fundamental concept that explains many aspects of atomic behavior and chemical properties. While Slater's rules offer a practical approach to estimating Z<sub>eff</sub>, more advanced computational techniques provide higher accuracy. Understanding Z<sub>eff</sub> and its influence on atomic size, ionization energy, electron affinity, electronegativity, and chemical reactivity is essential for grasping the underlying principles of chemistry and atomic physics. Further exploration into more advanced computational methods will provide a deeper understanding of the subtle nuances involved in calculating and applying Z<sub>eff</sub> in different contexts. The continued development and refinement of these methods are critical for advancements in various fields, including materials science, drug discovery, and the study of chemical reactions.
Latest Posts
Latest Posts
-
If A Pea Plant Shows A Recessive Phenotype
Mar 15, 2025
-
Is A Patent A Current Asset
Mar 15, 2025
-
Why Are Producers So Important To An Ecosystem
Mar 15, 2025
-
Difference Between Interest Groups And Political Parties
Mar 15, 2025
-
The Division Of The Cell Nucleus Is Called
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Effective Nuclear Charge Zeff Is Defined As . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
