During Boiling The Temperature Of A Liquid
News Leon
Apr 07, 2025 · 6 min read
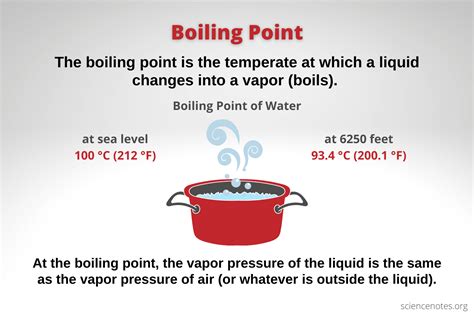
Table of Contents
During Boiling: Understanding the Temperature of a Liquid
Boiling is a familiar process, crucial in countless everyday tasks from cooking pasta to generating electricity. Yet, the precise mechanics of boiling, especially the temperature behavior of the liquid during this phase transition, often remain misunderstood. This article delves deep into the physics of boiling, explaining the temperature dynamics involved and dispelling common misconceptions. We will explore the factors influencing boiling point, the relationship between temperature and heat input, and the significance of boiling in various applications.
What Happens During Boiling?
Boiling, or ebullition, is the rapid vaporization of a liquid, occurring when its vapor pressure equals the surrounding atmospheric pressure. This means that the pressure exerted by the liquid's vapor trying to escape is sufficient to overcome the external pressure pushing down on it. This process isn't uniform; it's characterized by the formation and rapid expansion of vapor bubbles within the liquid.
Key Characteristics of Boiling:
-
Temperature Remains Constant: A crucial aspect of boiling is that, under constant pressure, the temperature of the liquid remains remarkably consistent throughout the entire boiling process. This constant temperature is known as the boiling point. Adding more heat doesn't increase the temperature; it simply increases the rate of vaporization.
-
Bubble Formation: The boiling process is visually distinct, marked by the formation and ascent of bubbles. These bubbles are initially tiny pockets of vapor that grow as they absorb more heat and rise to the surface, eventually bursting and releasing vapor into the air. The location of bubble nucleation (where the bubbles first form) is influenced by factors like surface roughness and the presence of impurities.
-
Heat Transfer: Boiling is a highly efficient method of heat transfer. The continuous formation and escape of vapor bubbles effectively transfer heat away from the liquid's surface, preventing the temperature from rising further above the boiling point.
Factors Affecting Boiling Point
While the boiling point of a liquid might seem like a fixed property, it's actually highly sensitive to several environmental factors:
1. Atmospheric Pressure:
This is perhaps the most significant influence. Lower atmospheric pressure leads to a lower boiling point, and vice-versa. At higher altitudes, where atmospheric pressure is reduced, liquids boil at lower temperatures. This is why it takes longer to cook food at high altitudes – the water boils at a lower temperature, meaning the food is subjected to less intense heat. Conversely, at higher pressures, the boiling point increases. Pressure cookers utilize this principle to cook food faster and more efficiently.
2. Impurities:
The presence of dissolved substances (impurities) in a liquid can elevate its boiling point. This phenomenon is known as boiling point elevation. The extent of the elevation is proportional to the concentration of dissolved particles. This is why saltwater boils at a slightly higher temperature than pure water.
3. Intermolecular Forces:
The strength of intermolecular forces between the molecules of a liquid significantly impacts its boiling point. Stronger forces require more energy to overcome, leading to a higher boiling point. For example, liquids with strong hydrogen bonds, like water, have relatively high boiling points compared to substances with weaker intermolecular forces.
4. Surface Tension:
Surface tension, the cohesive force between liquid molecules at the surface, can affect bubble formation and thus boiling behavior. Liquids with high surface tension may exhibit delayed or uneven boiling, sometimes leading to superheating (discussed in more detail later).
The Role of Heat Energy During Boiling
It's crucial to understand the distinction between temperature and heat. While temperature measures the average kinetic energy of molecules, heat represents the total energy transfer. During boiling, added heat energy isn't increasing the temperature of the liquid but rather providing the energy needed to overcome the intermolecular forces and transition the liquid molecules into the gaseous phase. This energy is used for the phase change, not for increasing kinetic energy (and therefore temperature).
This concept is often visualized using a heating curve. As heat is added to a liquid, its temperature rises until it reaches the boiling point. At this point, the temperature plateaus while the liquid boils, even as heat continues to be added. Only after all the liquid has vaporized will the temperature of the resulting gas begin to increase again.
Nucleation and Bubble Formation: A Closer Look
The initiation of bubble formation, termed nucleation, is a complex process significantly influenced by the presence of imperfections on the heating surface. These imperfections, such as scratches, cavities, or even dissolved gases, act as nucleation sites, providing surfaces for vapor bubbles to form more easily. Without these sites, the liquid might superheat.
Superheating: This occurs when a liquid is heated above its boiling point without boiling. This is typically unstable and will eventually result in a sudden, potentially violent, burst of boiling as numerous bubbles form rapidly. This is why it's generally recommended to use a slightly rough surface for heating liquids to avoid superheating.
Boiling in Different Contexts
Boiling plays a crucial role in diverse applications across science and industry:
-
Cooking: Boiling is fundamental to many culinary techniques, used to cook food, sterilize utensils, and extract flavors.
-
Power Generation: In power plants, water is boiled to generate steam, which then drives turbines to produce electricity. This is a key process in both fossil fuel and nuclear power generation.
-
Chemical Processes: Boiling is employed in many chemical processes for separation and purification techniques like distillation.
-
Refrigeration: Boiling is used in refrigeration cycles, where a refrigerant boils at a low temperature to absorb heat and cool the surrounding environment.
Misconceptions about Boiling
Several common misconceptions about boiling need clarification:
-
Boiling is faster than simmering: While boiling does lead to faster cooking, it's not always the most efficient method. Simmering can be more effective for delicate dishes, as it applies a gentler, more even heat.
-
Higher heat leads to faster cooking: While more heat increases the rate of boiling, it doesn't necessarily lead to faster overall cooking. Once the boiling point is reached, adding more heat only speeds the vaporization rate, not the cooking process itself. This is why efficient cooking often involves achieving a consistent boil rather than using excessively high heat.
-
All liquids boil at 100°C (212°F): This is true only for pure water at standard atmospheric pressure. The boiling point varies significantly with changes in pressure and the presence of impurities, as discussed above.
Conclusion: Understanding Boiling for Improved Applications
Boiling, a seemingly simple process, reveals rich complexities when examined closely. Understanding the factors influencing boiling point, the constant temperature maintained during boiling under constant pressure, and the role of heat energy is crucial for optimizing numerous applications, from everyday cooking to advanced industrial processes. By dispelling misconceptions and appreciating the intricate physics involved, we can harness the power of boiling more effectively and efficiently. The information presented here provides a foundational understanding of boiling and its implications across various domains, highlighting the importance of understanding the subtle yet significant details that govern this fundamental phase transition. Further exploration into specialized areas like nucleate boiling, film boiling, and critical heat flux will reveal even more about this dynamic and essential process.
Latest Posts
Latest Posts
-
Can A Substance Contract On Heating Give An Example
Apr 08, 2025
-
Which Three Organelles Are Not Surrounded By Membranes
Apr 08, 2025
-
Red Blood Cells Placed In Distilled Water Will
Apr 08, 2025
-
Arrange The Following Species By Decreasing Atomic Radii
Apr 08, 2025
-
Predict The Product For The Following Reaction Sequence
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about During Boiling The Temperature Of A Liquid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.