Does A Gas Have Definite Volume
News Leon
Mar 20, 2025 · 5 min read
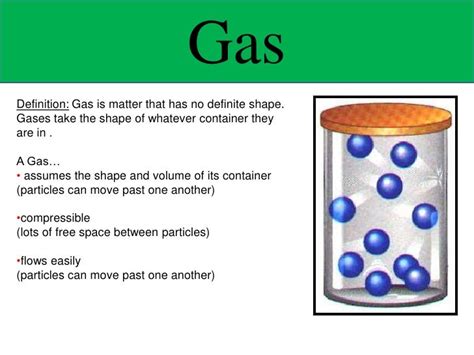
Table of Contents
Does a Gas Have a Definite Volume? Understanding Gas Behavior
The question of whether a gas has a definite volume is a fundamental concept in chemistry and physics. The short answer is no, a gas does not have a definite volume. Unlike solids and liquids, which maintain a relatively fixed shape and volume, gases are highly compressible and will expand or contract to fill the container they occupy. This behavior is governed by the properties of gas molecules and the forces acting upon them. This article will delve deeper into the characteristics of gases, exploring the factors that influence their volume and providing a comprehensive understanding of this key concept.
Understanding the Kinetic Molecular Theory of Gases
To truly grasp the answer, we must understand the Kinetic Molecular Theory of Gases (KMT). This theory provides a microscopic model to explain the macroscopic behavior of gases. The key postulates of the KMT are:
- Gases are composed of tiny particles (atoms or molecules) that are in constant, random motion. These particles are in a state of perpetual movement, colliding with each other and the walls of their container.
- The volume of the gas particles themselves is negligible compared to the volume of the container. This means the space occupied by the gas particles is insignificant compared to the total volume of the gas.
- There are no attractive or repulsive forces between gas particles. This is an idealization; real gases do experience intermolecular forces, but these are often weak enough to be neglected in many situations.
- Collisions between gas particles and the container walls are perfectly elastic. This means that no kinetic energy is lost during collisions.
- The average kinetic energy of the gas particles is directly proportional to the absolute temperature. Higher temperatures mean faster-moving particles and greater kinetic energy.
These postulates explain why gases expand to fill their containers. Because the gas particles are in constant motion and there are negligible intermolecular forces, they move freely and independently, spreading out to occupy the entire available space. Therefore, the volume of the gas is determined by the volume of the container, not an inherent property of the gas itself.
Factors Affecting Gas Volume
Several factors influence the volume of a gas. These are intricately linked and described by the Ideal Gas Law, a fundamental equation in chemistry:
PV = nRT
Where:
- P represents pressure
- V represents volume
- n represents the number of moles of gas
- R represents the ideal gas constant
- T represents temperature (in Kelvin)
Let's analyze how each factor affects volume:
Pressure (P)
Pressure is the force exerted by gas particles per unit area on the walls of the container. Increasing the pressure reduces the volume of the gas, and vice versa. This is because increased pressure forces the gas particles closer together, reducing the overall volume. Imagine squeezing a balloon; you're increasing the pressure and decreasing the volume.
Temperature (T)
Temperature is a measure of the average kinetic energy of the gas particles. Increasing the temperature increases the volume of the gas, provided the pressure remains constant. This is because higher temperatures lead to faster-moving particles, which collide more forcefully with the container walls and require a larger volume to maintain the same pressure.
Number of Moles (n)
The number of moles represents the amount of gas present. Increasing the number of moles increases the volume of the gas, assuming the pressure and temperature remain constant. More gas particles mean more collisions with the container walls, requiring more space to maintain the existing pressure.
Ideal Gas Constant (R)
The ideal gas constant (R) is a proportionality constant that relates the pressure, volume, temperature, and number of moles of a gas. It's a fixed value that doesn't change with the conditions of the gas.
Real Gases vs. Ideal Gases
The Ideal Gas Law provides a good approximation for the behavior of many gases under normal conditions. However, it's important to remember that it's a model, and real gases deviate from ideal behavior under certain conditions, such as high pressure and low temperature.
At high pressures, the volume of the gas particles themselves becomes significant compared to the volume of the container. This is because the particles are forced closer together, reducing the free space they can occupy. At low temperatures, intermolecular forces become more significant, causing the gas particles to attract each other and reduce the volume.
These deviations from ideal behavior are accounted for by more complex equations of state, such as the van der Waals equation, which incorporates corrections for intermolecular forces and the finite volume of gas particles.
Applications and Real-World Examples
Understanding the concept of gas volume and its relationship to pressure and temperature has numerous practical applications:
- Weather forecasting: Atmospheric pressure and temperature directly influence weather patterns. Changes in these factors affect the volume of air masses, leading to changes in wind speed, cloud formation, and precipitation.
- Automotive engineering: Internal combustion engines rely on the controlled expansion and compression of gases to generate power. The volume of the gases within the engine cylinders is carefully regulated to optimize performance.
- Aerospace engineering: The behavior of gases at high altitudes and low pressures is critical in aircraft design and operation. Understanding gas expansion and contraction is vital for controlling altitude and maintaining cabin pressure.
- Industrial processes: Many industrial processes involve the use of gases, such as in chemical reactions and material processing. Precise control over gas volume is essential for efficient and safe operation.
- Diving and underwater exploration: Gas volume changes significantly with depth due to changes in pressure. Divers need to understand these changes to avoid decompression sickness.
Conclusion
In conclusion, a gas does not possess a definite volume. Its volume is dictated by the container it occupies, and it expands or contracts to fill that space. The kinetic molecular theory of gases explains this behavior, and the ideal gas law mathematically describes the relationship between volume, pressure, temperature, and the number of moles. While the ideal gas law is a useful approximation, real gases deviate from ideal behavior under certain conditions. Understanding these principles is crucial in numerous scientific, engineering, and industrial applications. The ability to predict and control gas volume is fundamental to numerous technologies and natural phenomena, underscoring the importance of understanding this fundamental concept.
Latest Posts
Latest Posts
-
Metals Are Good Conductors Of Electricity
Mar 20, 2025
-
In Which Layer Of The Atmosphere Does Most Weather Occur
Mar 20, 2025
-
An Atom That Gains An Electron Is Called
Mar 20, 2025
-
The Following Figure Shows An Example Of
Mar 20, 2025
-
What Is Difference Between Mcg And Mg
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Does A Gas Have Definite Volume . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
