Conductivity Of Conductors Semiconductors And Insulators
News Leon
Mar 27, 2025 · 6 min read
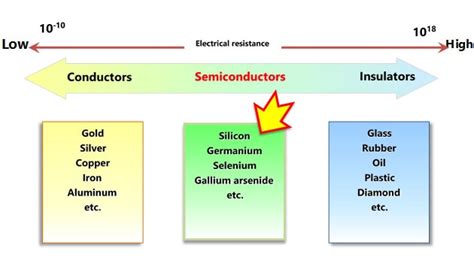
Table of Contents
Conductivity of Conductors, Semiconductors, and Insulators: A Deep Dive
Understanding the conductivity of materials is fundamental to electronics and materials science. The ability of a material to conduct electricity hinges on its atomic structure and the behavior of its electrons. This article will explore the conductivity of conductors, semiconductors, and insulators, examining the underlying mechanisms and providing practical examples.
What is Electrical Conductivity?
Electrical conductivity is a measure of a material's ability to allow the flow of electric current. It's the inverse of electrical resistivity, meaning a material with high conductivity has low resistivity and vice versa. The flow of current is essentially the movement of charge carriers, primarily electrons, within the material. The ease with which these charge carriers can move determines the material's conductivity. This ease of movement is heavily influenced by the material's electronic structure and its temperature.
Units of Conductivity
Electrical conductivity (σ) is typically measured in Siemens per meter (S/m). A higher value of σ indicates a better conductor. Sometimes, you'll encounter conductivity expressed as its inverse, resistivity (ρ), measured in ohm-meters (Ω·m).
Conductors: The Free Electron Sea
Conductors are materials that readily allow the flow of electric current. Their characteristic feature is the presence of a large number of free electrons, which are not bound to any particular atom. These free electrons form a "sea" of electrons that can move easily throughout the material when an electric field is applied.
Atomic Structure and Conductivity in Conductors
The atomic structure of conductors plays a crucial role in their high conductivity. Typically, conductors have one or two loosely bound valence electrons that are easily detached from their atoms. These electrons become delocalized and contribute to the "sea" of free electrons responsible for current flow. Examples include metals like copper, silver, gold, aluminum, and iron. These metals have a relatively simple crystal structure, with closely packed atoms facilitating the movement of electrons.
Factors Affecting Conductivity in Conductors
Several factors can affect the conductivity of conductors:
- Temperature: As temperature increases, the vibrations of the atoms in the lattice increase, scattering the electrons and hindering their movement. This results in a decrease in conductivity.
- Impurities: The presence of impurities in a conductor can also reduce conductivity. Impurities disrupt the regular crystal lattice, creating scattering centers for the electrons.
- Crystal Structure: Defects in the crystal structure, such as dislocations and grain boundaries, can impede electron flow, reducing conductivity.
Semiconductors: A Bridge Between Conductors and Insulators
Semiconductors occupy a middle ground between conductors and insulators in terms of their electrical conductivity. Their conductivity is significantly lower than conductors but higher than insulators. Crucially, their conductivity can be dramatically altered by factors like temperature, doping (adding impurities), and light exposure.
Intrinsic Semiconductors: Pure Silicon and Germanium
Intrinsic semiconductors are pure materials like silicon (Si) and germanium (Ge) with a relatively small number of free charge carriers at room temperature. The conductivity is determined by the thermal generation of electron-hole pairs. At absolute zero temperature, an intrinsic semiconductor behaves as an insulator. As temperature rises, more electrons gain enough energy to break free from their covalent bonds, creating electron-hole pairs and increasing conductivity.
Extrinsic Semiconductors: Doping for Enhanced Conductivity
Extrinsic semiconductors are created by intentionally adding impurities (dopants) to an intrinsic semiconductor to modify its conductivity. There are two types of doping:
- N-type doping: Adding pentavalent impurities (e.g., phosphorus, arsenic) introduces extra electrons, making the semiconductor negatively charged. These extra electrons become the majority charge carriers.
- P-type doping: Adding trivalent impurities (e.g., boron, aluminum) creates "holes" – the absence of an electron in the valence band. These holes act as positive charge carriers, becoming the majority charge carriers.
Doping drastically increases the conductivity of semiconductors, making them suitable for electronic devices.
Factors Affecting Semiconductors' Conductivity
Besides temperature and doping, other factors influence the conductivity of semiconductors:
- Light: Light can generate electron-hole pairs, increasing conductivity (photoconductivity).
- Electric Field: A strong electric field can increase the mobility of charge carriers, enhancing conductivity.
Insulators: Resisting the Flow of Current
Insulators are materials that strongly resist the flow of electric current. Their conductivity is extremely low because they have very few free electrons. Their valence electrons are tightly bound to their atoms, making it difficult for them to move freely.
Atomic Structure and Insulating Properties
Insulators typically have a large energy gap (band gap) between the valence band (where electrons are bound) and the conduction band (where electrons are free to move). A significant amount of energy is needed to excite an electron from the valence band to the conduction band. This makes it very difficult for current to flow at normal temperatures. Examples of insulators include rubber, glass, plastic, and ceramics.
Breakdown Voltage: The Limit of Insulation
Even insulators can conduct electricity if a sufficiently high voltage is applied. This is because the strong electric field can overcome the energy gap and strip electrons from their atoms, leading to electrical breakdown. This is a destructive process and can damage the insulator.
Comparing Conductivity Across Material Types
The table below summarizes the key differences in conductivity between conductors, semiconductors, and insulators:
| Feature | Conductors | Semiconductors | Insulators |
|---|---|---|---|
| Conductivity | High | Moderate, variable | Very Low |
| Charge Carriers | Free electrons | Electrons and holes | Very few free charge carriers |
| Band Gap | Overlapping valence and conduction bands | Small band gap | Large band gap |
| Temperature Dependence | Decreases with increasing temperature | Increases with increasing temperature | Relatively unaffected by temperature |
| Examples | Copper, silver, gold, aluminum | Silicon, germanium | Rubber, glass, plastic, ceramics |
Applications of Conductors, Semiconductors, and Insulators
The unique electrical properties of conductors, semiconductors, and insulators make them essential components in countless electronic devices and technologies:
Conductors: Used in electrical wiring, circuits, and components where efficient current flow is required.
Semiconductors: Form the basis of modern electronics, including transistors, integrated circuits (ICs), diodes, and solar cells. Their ability to control current flow enables complex computations and signal processing.
Insulators: Used for insulation in electrical wiring, preventing short circuits and ensuring safety. They are also found in many components as protective layers.
Advanced Concepts and Future Directions
Research in materials science continually pushes the boundaries of conductivity. Areas of ongoing investigation include:
- High-temperature superconductors: Materials that exhibit zero electrical resistance below a critical temperature.
- Graphene and other two-dimensional materials: Exploring their potential for high conductivity and novel electronic applications.
- Organic semiconductors: Developing environmentally friendly and flexible electronics.
- Topological insulators: Materials that are insulating in their bulk but conductive on their surface. These have potential applications in spintronics and quantum computing.
Conclusion
The conductivity of materials is a fundamental property that underpins the functioning of countless technologies. Understanding the differences between conductors, semiconductors, and insulators, and the factors influencing their conductivity, is essential for anyone working in electronics, materials science, or related fields. Continued research and innovation in materials science will undoubtedly lead to further advancements in the development of new materials with enhanced conductivity and improved performance in electronic applications. The exploration of new materials and their unique conductive properties opens up exciting possibilities for future technological advancements.
Latest Posts
Latest Posts
-
The Si Unit Of Power Is
Mar 30, 2025
-
Where Are Unmyelinated Nerve Fibers Surrounded By Schwann Cells
Mar 30, 2025
-
A Square Wire Loop With 2 00 M Sides
Mar 30, 2025
-
What Percent Of 752 Is 25
Mar 30, 2025
-
A Fully Loaded Slow Moving Freight Elevator
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Conductivity Of Conductors Semiconductors And Insulators . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
