Concentration Of Hydrogen Ions In Water With Ph Of 7
News Leon
Apr 01, 2025 · 6 min read
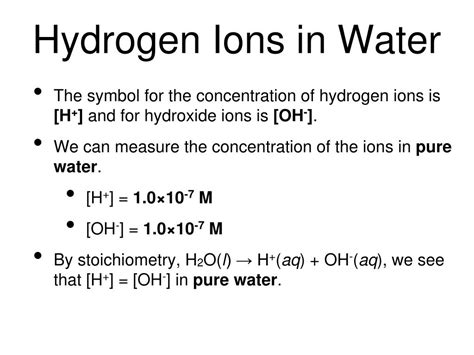
Table of Contents
Concentration of Hydrogen Ions in Water with a pH of 7: A Deep Dive
The pH scale, a logarithmic measure of hydrogen ion (H⁺) concentration, is fundamental to understanding the acidity or alkalinity of aqueous solutions. Pure water, at 25°C, holds a special place on this scale, boasting a pH of precisely 7. This seemingly simple number masks a fascinating interplay of chemical equilibrium and ion concentrations, which we will explore in detail. This article will delve into the concentration of hydrogen ions in water with a pH of 7, examining the underlying chemistry, implications for biological systems, and the significance of maintaining this delicate balance.
Understanding the pH Scale and its Logarithmic Nature
The pH scale ranges from 0 to 14, with 7 representing neutrality. Values below 7 indicate acidity, while values above 7 signify alkalinity. Crucially, the pH scale is logarithmic, meaning each whole number change represents a tenfold difference in H⁺ concentration. This means a solution with a pH of 6 has ten times the H⁺ concentration of a solution with a pH of 7, and a solution with a pH of 5 has one hundred times the H⁺ concentration of a solution with a pH of 7.
This logarithmic nature is essential for encompassing the vast range of H⁺ concentrations encountered in various natural and man-made systems. From highly acidic solutions found in battery acid to highly alkaline solutions like drain cleaner, the pH scale provides a convenient and manageable way to express these differences.
The Autoionization of Water: The Source of H⁺ and OH⁻ Ions
The neutral pH of pure water (7 at 25°C) arises from the autoionization of water molecules. Water molecules (H₂O) are inherently amphoteric, meaning they can act as both acids and bases. In a small fraction of water molecules, a proton (H⁺) transfers from one water molecule to another, forming a hydronium ion (H₃O⁺) and a hydroxide ion (OH⁻).
This process can be represented by the following equilibrium reaction:
2H₂O(l) ⇌ H₃O⁺(aq) + OH⁻(aq)
The equilibrium constant for this reaction, denoted as Kw (the ion product constant for water), is crucial. At 25°C, Kw has a value of approximately 1.0 x 10⁻¹⁴. This constant represents the product of the concentrations of H₃O⁺ and OH⁻ ions:
Kw = [H₃O⁺][OH⁻] = 1.0 x 10⁻¹⁴
In pure water, the concentrations of H₃O⁺ and OH⁻ ions are equal, reflecting the neutrality of the solution. Therefore, we can calculate the concentration of H⁺ (approximated by H₃O⁺ for simplicity):
[H₃O⁺] = [OH⁻] = √Kw = √(1.0 x 10⁻¹⁴) = 1.0 x 10⁻⁷ M
This calculation demonstrates that the concentration of hydrogen ions (H⁺) in pure water at 25°C is 1.0 x 10⁻⁷ moles per liter (M). This is the fundamental basis for the pH of 7. It is important to note that this is an approximation because the activity of ions differs from concentration, particularly at higher concentrations.
Temperature Dependence of Kw and pH
It’s important to emphasize that the Kw value and consequently, the pH of pure water, are temperature-dependent. At higher temperatures, the autoionization of water increases, leading to a higher Kw and a slightly lower pH (still near 7, but not exactly 7). At lower temperatures, the opposite occurs. This temperature dependence is a crucial aspect to consider when working with pH measurements in different environmental conditions.
Implications for Biological Systems: Maintaining pH Homeostasis
The pH of 7 is vital for the proper functioning of biological systems. Many biological processes are exquisitely sensitive to even small changes in pH. Enzymes, the biological catalysts that drive countless metabolic reactions, have optimal pH ranges within which they function efficiently. Deviation from these optimal ranges can lead to decreased enzyme activity, potentially disrupting cellular processes and impacting overall organismal health.
Maintaining a stable pH, often referred to as pH homeostasis, is critical for cellular function. Living organisms employ sophisticated buffering systems to resist changes in pH. These buffer systems consist of weak acids and their conjugate bases, which can absorb excess H⁺ or OH⁻ ions, thus minimizing pH fluctuations. The bicarbonate buffer system, involving carbonic acid (H₂CO₃) and bicarbonate ions (HCO₃⁻), is a prime example of such a buffering mechanism in human blood.
Measuring pH: Methods and Applications
Precise pH measurement is critical in many scientific, industrial, and environmental contexts. Several methods are commonly employed, including:
-
pH Meters: These electronic instruments use a pH-sensitive electrode to measure the potential difference between the electrode and a reference electrode. The potential difference is directly related to the pH of the solution. pH meters are widely used for accurate and rapid pH determination.
-
pH Indicators: These are substances that change color depending on the pH of the solution. Litmus paper, a common example, is used for quick, qualitative pH assessments. More sophisticated pH indicators are used in titrations and other analytical methods.
-
Spectrophotometry: This technique measures the absorbance of light by a solution at specific wavelengths. Certain compounds exhibit changes in absorbance with varying pH, enabling spectrophotometric pH determination.
Applications of pH Measurement
Precise pH control and monitoring are essential in diverse applications, including:
-
Water Quality Monitoring: pH plays a crucial role in water quality. Maintaining a suitable pH range is essential for aquatic life. Monitoring pH helps in detecting pollution and ensuring water safety.
-
Agriculture: Soil pH significantly impacts plant growth. Monitoring and adjusting soil pH is crucial for optimal crop yield.
-
Medicine: Blood pH is meticulously maintained within a narrow range. Deviation from this range can lead to serious medical conditions. Monitoring and controlling blood pH is vital in healthcare.
-
Industry: Many industrial processes require precise pH control, such as in the production of pharmaceuticals, food processing, and wastewater treatment.
Deviations from pH 7: Acidity and Alkalinity
While a pH of 7 represents neutrality, deviations from this value are often encountered and are significant in various contexts:
-
Acidity (pH < 7): Increased H⁺ concentration leads to acidity. Acidity can be harmful to many biological systems, corroding materials, and impacting environmental health. Acid rain, caused by atmospheric pollutants, is a stark example of the environmental consequences of acidity.
-
Alkalinity (pH > 7): Increased OH⁻ concentration leads to alkalinity. While some alkaline environments are essential (e.g., certain cleaning products), excessive alkalinity can also be harmful to living organisms and damaging to materials.
Conclusion: The Significance of pH 7
The concentration of hydrogen ions in water with a pH of 7, precisely 1.0 x 10⁻⁷ M at 25°C, is not merely a chemical curiosity; it’s a fundamental aspect of numerous natural and man-made systems. Understanding the autoionization of water, the logarithmic nature of the pH scale, and the biological implications of pH is crucial for comprehending various scientific, environmental, and industrial processes. Maintaining pH homeostasis, whether in biological systems or industrial processes, is often critical for optimal function and preventing detrimental consequences. The seemingly simple pH of 7 in pure water is a cornerstone of chemistry and biology, underscoring the power of subtle ion concentrations in shaping our world.
Latest Posts
Latest Posts
-
Complete The Complementary Strand Of Dna
Apr 02, 2025
-
Is Carbon Dioxide A Pure Substance Or Mixture
Apr 02, 2025
-
The Given Reaction Proceeds In Two Parts
Apr 02, 2025
-
Which Of The Following Combinations Is Correct
Apr 02, 2025
-
How Do You Find The Boiling Point Of A Solution
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Concentration Of Hydrogen Ions In Water With Ph Of 7 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
