Chemical Formula Of Sodium And Oxygen
News Leon
Mar 17, 2025 · 5 min read
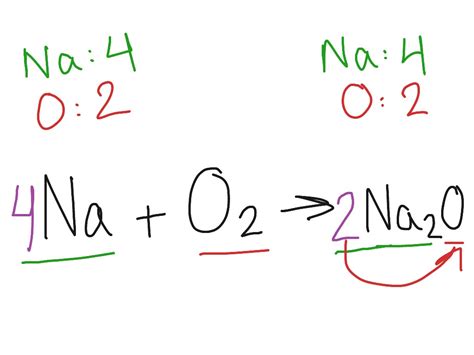
Table of Contents
The Chemical Formula of Sodium and Oxygen: A Deep Dive into Na₂O and Beyond
Sodium (Na) and oxygen (O) are two highly reactive elements that readily combine to form a stable ionic compound: sodium oxide (Na₂O). Understanding the chemical formula Na₂O and the intricacies of the reaction between sodium and oxygen is crucial for comprehending a vast range of chemical processes, from everyday occurrences to industrial applications. This article will explore the formation of sodium oxide, its properties, its applications, and related compounds, providing a comprehensive overview of the chemistry involved.
The Formation of Sodium Oxide (Na₂O)
The reaction between sodium and oxygen is a vigorous exothermic process, meaning it releases a significant amount of heat. Sodium, an alkali metal, readily loses one electron to achieve a stable electron configuration, becoming a positively charged sodium ion (Na⁺). Oxygen, a nonmetal, readily gains two electrons to achieve a stable electron configuration, becoming a negatively charged oxide ion (O²⁻). The electrostatic attraction between these oppositely charged ions is the driving force behind the formation of sodium oxide.
The Balanced Chemical Equation
The balanced chemical equation representing the reaction is:
4Na(s) + O₂(g) → 2Na₂O(s)
This equation signifies that four atoms of solid sodium react with one molecule of gaseous oxygen to produce two formula units of solid sodium oxide. The coefficients (4, 1, and 2) ensure that the number of atoms of each element is equal on both sides of the equation, adhering to the law of conservation of mass.
The Ionic Bond in Na₂O
The bond between the sodium and oxide ions in Na₂O is an ionic bond. This type of bond arises from the electrostatic attraction between the positively charged sodium cation (Na⁺) and the negatively charged oxide anion (O²⁻). The strong electrostatic forces hold the ions together in a crystalline lattice structure. This structure contributes to the properties of sodium oxide, such as its high melting and boiling points.
Properties of Sodium Oxide (Na₂O)
Sodium oxide is a white, hygroscopic solid. "Hygroscopic" means it readily absorbs moisture from the air. This property is due to the strong attraction between the polar water molecules and the sodium and oxide ions. Exposure to air leads to the formation of sodium hydroxide (NaOH) through the following reaction:
Na₂O(s) + H₂O(l) → 2NaOH(aq)
This reaction is another example of an exothermic process. The resulting sodium hydroxide solution is highly alkaline (basic), possessing a high pH value.
Other key properties of sodium oxide include:
- High melting point: The strong ionic bonds require a significant amount of energy to overcome, resulting in a high melting point.
- Solubility: It is soluble in water, reacting readily to form sodium hydroxide.
- Reactivity: It is a highly reactive compound, readily reacting with acids and water.
- Crystalline Structure: It possesses a specific crystalline structure, typically a cubic anti-fluorite structure.
Applications of Sodium Oxide (Na₂O)
Despite its reactivity, sodium oxide finds applications in several industrial processes. It is primarily used as:
- A precursor for other sodium compounds: Its high reactivity makes it a valuable starting material for synthesizing other sodium-containing compounds.
- A component in glass manufacturing: Although not directly added, sodium oxide is a crucial component formed during the glass-making process. Sodium carbonate (Na₂CO₃) is often used, which decomposes at high temperatures to release Na₂O, lowering the melting point of silica (SiO₂) and improving the workability of the glass. This contributes to the properties and quality of the final glass product.
- A drying agent: Its hygroscopic nature allows it to absorb moisture, making it useful in certain drying applications. However, due to its reactivity with water, its application is limited.
- Ceramic industry: It plays a role in the production of certain types of ceramics, where its ability to interact with other oxides can influence the final product's properties.
Related Compounds and Reactions
Understanding the chemistry of sodium oxide necessitates exploring related compounds and reactions involving sodium and oxygen.
Sodium Peroxide (Na₂O₂)
Sodium peroxide (Na₂O₂) is another compound formed from the reaction of sodium and oxygen, particularly under conditions with an excess of oxygen. The chemical equation is:
2Na(s) + O₂(g) → Na₂O₂(s)
Sodium peroxide contains the peroxide ion (O₂²⁻), a species with an oxygen-oxygen single bond and a -1 charge on each oxygen atom. Unlike sodium oxide, sodium peroxide reacts with water to produce hydrogen peroxide (H₂O₂) and sodium hydroxide:
Na₂O₂(s) + 2H₂O(l) → 2NaOH(aq) + H₂O₂(aq)
Sodium Superoxide (NaO₂)
Under specific conditions, particularly at high pressures and low temperatures, sodium superoxide (NaO₂) can form. This compound contains the superoxide ion (O₂⁻). This ion has an oxygen-oxygen bond order between a single and a double bond and a -1/2 charge per oxygen atom. The reaction is more complex and often involves catalytic influences.
Reactions with Acids
Sodium oxide readily reacts with acids, undergoing a neutralization reaction. For example, its reaction with hydrochloric acid (HCl) produces sodium chloride (NaCl) and water:
Na₂O(s) + 2HCl(aq) → 2NaCl(aq) + H₂O(l)
This type of reaction is highly exothermic and often rapid. Similar reactions occur with other strong acids.
Safety Precautions
Sodium and sodium oxide are highly reactive substances. Direct contact with skin or eyes can cause severe burns. Inhaling sodium oxide dust can also be harmful to the respiratory system. Always handle these substances with appropriate safety precautions, including wearing protective gear such as gloves, goggles, and a lab coat. Work should be carried out in a well-ventilated area or under a fume hood to prevent inhalation of hazardous materials.
Conclusion
The chemical formula of sodium and oxygen, Na₂O, represents a fundamental ionic compound formed through a highly exothermic reaction. Understanding the properties, applications, and related compounds of sodium oxide provides a deeper understanding of the fundamental principles of ionic bonding, reactivity, and the chemical behavior of alkali metals and oxygen. The information provided here underscores the importance of proper handling and safety procedures when working with these reactive materials. Further exploration into the broader context of sodium chemistry, including its role in biological systems and industrial processes, would enhance the comprehensive understanding of this vital element and its compounds. This detailed understanding is crucial for various scientific disciplines, from materials science and chemical engineering to environmental science and medicine.
Latest Posts
Latest Posts
-
Lines Of Symmetry On A Trapezoid
Mar 18, 2025
-
Two Same Words With Different Meanings
Mar 18, 2025
-
Select The Correct Statement About Equilibrium
Mar 18, 2025
-
Draw The Major Product Of The Following Reaction
Mar 18, 2025
-
A Wire Loop Of Radius 10 Cm And Resistance
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Chemical Formula Of Sodium And Oxygen . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
