Ch3 Ch2 O Ch2 Ch2 Ch3
News Leon
May 05, 2025 · 5 min read
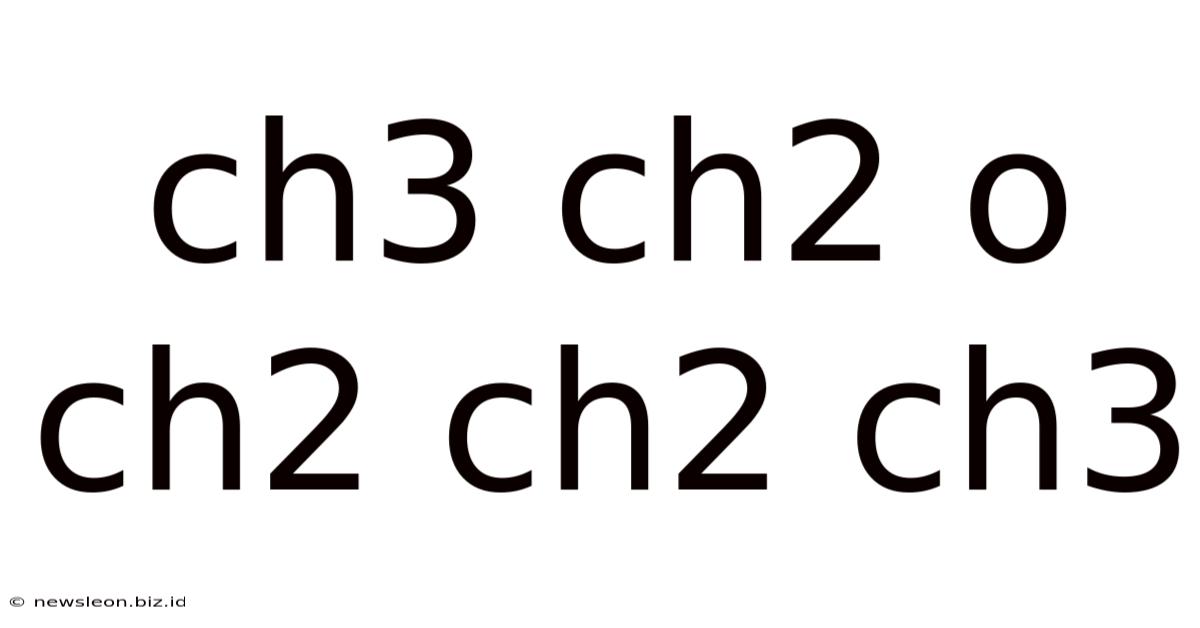
Table of Contents
Understanding the Organic Compound: CH3CH2OCH2CH2CH3
Introduction:
The chemical formula CH3CH2OCH2CH2CH3 represents ethyl propyl ether, also known as 1-ethoxypropane. This organic compound belongs to the ether family, characterized by an oxygen atom bonded to two alkyl groups. Understanding its structure, properties, synthesis, and applications is crucial in various fields, from organic chemistry to industrial applications. This comprehensive article delves into the intricacies of ethyl propyl ether, covering its key characteristics and significance.
Structural Features and Isomerism
Ethyl propyl ether's structure is relatively straightforward. It consists of an ether functional group (-O-) linking an ethyl group (CH3CH2-) and a propyl group (CH3CH2CH2-). This specific arrangement leads to unique physical and chemical properties compared to other ethers.
Understanding the Ether Linkage:
The oxygen atom in the ether linkage plays a critical role in determining the compound's reactivity. The oxygen's lone pairs of electrons contribute to its polarity and ability to interact with other molecules. This polarity influences its solubility and boiling point.
Isomers and Constitutional Isomers:
Ethyl propyl ether is just one example within a broader class of ethers. It exhibits isomerism, specifically constitutional isomerism. This means other ethers exist with the same molecular formula (C5H12O) but with different structural arrangements. For example, n-propyl ethyl ether and methyl n-butyl ether are constitutional isomers of ethyl propyl ether. Understanding these isomeric forms allows for a more nuanced understanding of their diverse properties.
Physical and Chemical Properties
The physical and chemical properties of ethyl propyl ether are strongly influenced by its structure and the presence of the ether functional group.
Boiling Point and Volatility:
Ethyl propyl ether possesses a relatively low boiling point compared to alcohols of similar molecular weight. This is due to the absence of hydrogen bonding, a strong intermolecular force present in alcohols. The weaker intermolecular forces (dipole-dipole interactions and London dispersion forces) in ethers result in lower boiling points and higher volatility.
Solubility:
The solubility of ethyl propyl ether in water is limited due to its relatively nonpolar nature. The alkyl chains dominate the molecule's character, decreasing its ability to form hydrogen bonds with water molecules. However, it exhibits good solubility in organic solvents. This behavior is consistent with "like dissolves like," a fundamental principle in solubility.
Reactivity:
Ethers are generally considered relatively unreactive compared to alcohols or aldehydes/ketones. However, under specific conditions, ethyl propyl ether can undergo reactions such as:
- Acid-catalyzed cleavage: Strong acids can cleave the ether linkage, breaking it down into smaller alcohol molecules. This reaction requires a high concentration of strong acid and elevated temperatures.
- Autoxidation: Upon exposure to air and light, ethers can slowly undergo autoxidation, forming potentially explosive peroxides. Therefore, proper storage and handling of ethyl propyl ether are crucial.
- Reactions with strong reducing agents: Although not as readily reactive as other functional groups, ethyl propyl ether can participate in reactions with strong reducing agents under specific conditions.
Synthesis of Ethyl Propyl Ether
The synthesis of ethyl propyl ether typically involves the Williamson ether synthesis. This reaction is a fundamental method for preparing ethers, relying on the reaction between an alkoxide ion and an alkyl halide.
Williamson Ether Synthesis in Detail:
The Williamson ether synthesis involves two main steps:
-
Formation of the alkoxide ion: A strong base, such as sodium hydroxide (NaOH), is used to deprotonate an alcohol, forming the corresponding alkoxide ion. For ethyl propyl ether synthesis, either ethanol or propanol could be used to generate the alkoxide.
-
SN2 reaction: The alkoxide ion then attacks an alkyl halide (e.g., propyl bromide or ethyl bromide) via an SN2 (Substitution Nucleophilic Bimolecular) mechanism. This reaction replaces the halide with the alkoxide group, forming the desired ether.
The choice of the alcohol and alkyl halide will determine the final product. Careful selection is essential to maximize the yield and minimize unwanted side reactions.
Applications of Ethyl Propyl Ether
Ethyl propyl ether, although not as widely used as some other ethers like diethyl ether, finds applications in several areas:
Solvent in Organic Chemistry:
Due to its relatively low boiling point and good solubility in organic compounds, ethyl propyl ether finds use as a solvent in various organic reactions and extractions. It serves as a medium to facilitate chemical transformations and isolate desired products.
Extraction of Natural Products:
It can be employed in the extraction of natural products from plant materials. The ether dissolves specific components, which can then be separated and purified.
Analytical Chemistry:
Ethyl propyl ether might have applications in analytical chemistry, such as in chromatography, serving as a solvent in sample preparation or mobile phases.
Safety and Handling
Handling ethyl propyl ether requires caution due to its volatility and potential for peroxide formation.
Flammability:
Ethyl propyl ether is highly flammable, requiring careful handling away from open flames or ignition sources. Adequate ventilation is crucial to prevent the accumulation of flammable vapors.
Peroxide Formation:
Exposure to air and light can lead to the formation of peroxides, which are explosive. Regular testing for peroxides and proper storage practices are essential to mitigate the risk of explosions.
Health Hazards:
Inhalation of ethyl propyl ether vapors can cause dizziness, headaches, and nausea. Skin contact can cause irritation, and eye contact may lead to severe irritation. Appropriate personal protective equipment (PPE), including gloves, goggles, and a respirator, should be used when handling this compound.
Conclusion:
Ethyl propyl ether (CH3CH2OCH2CH2CH3), although less prominent than some other ethers, presents a fascinating example of an organic compound with unique structural features and properties. Its synthesis through the Williamson ether synthesis provides valuable insights into the reactivity of alkoxides and alkyl halides. Understanding its physical and chemical properties, including its reactivity, solubility, and volatility, is essential for its safe and effective application in organic chemistry, extraction processes, and potentially in analytical techniques. Always remember the importance of safety precautions when working with this or any other organic compound, especially regarding its flammability and the potential for peroxide formation. Furthermore, the exploration of its isomeric forms and the variations in their properties contribute to a comprehensive understanding of this family of organic compounds. The detailed examination of its synthesis methods allows for a deeper grasp of organic reaction mechanisms and their implications in chemical synthesis. Finally, the discussion of its potential applications highlights its role in various fields, reinforcing its significance within the broader context of organic chemistry and its practical implications.
Latest Posts
Related Post
Thank you for visiting our website which covers about Ch3 Ch2 O Ch2 Ch2 Ch3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.