Calcium Carbonate And Hydrochloric Acid Balanced Equation
News Leon
Apr 06, 2025 · 5 min read
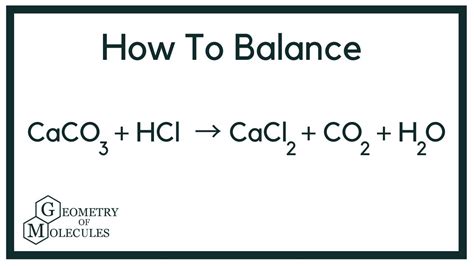
Table of Contents
Calcium Carbonate and Hydrochloric Acid: A Balanced Equation and Beyond
The reaction between calcium carbonate (CaCO₃) and hydrochloric acid (HCl) is a classic example of an acid-base reaction, frequently encountered in chemistry classrooms and relevant to various industrial processes and geological phenomena. Understanding this reaction, from balancing its equation to exploring its applications and implications, is crucial for a solid grasp of chemical principles. This article delves deep into the intricacies of this reaction, examining its balanced equation, the underlying mechanisms, practical applications, and safety considerations.
The Balanced Chemical Equation
The reaction between calcium carbonate and hydrochloric acid produces calcium chloride, water, and carbon dioxide. The unbalanced equation is:
CaCO₃ + HCl → CaCl₂ + H₂O + CO₂
To balance this equation, we need to ensure that the number of atoms of each element is the same on both sides of the equation. This is achieved by adjusting the stoichiometric coefficients:
CaCO₃ + 2HCl → CaCl₂ + H₂O + CO₂
This balanced equation shows that one mole of calcium carbonate reacts with two moles of hydrochloric acid to produce one mole of calcium chloride, one mole of water, and one mole of carbon dioxide. This stoichiometric ratio is essential for quantitative analysis and predictions of the reaction's outcome.
Understanding the Reaction Mechanism
The reaction proceeds in two main steps:
Step 1: Acid-Base Reaction
The hydrochloric acid, a strong acid, donates a proton (H⁺) to the calcium carbonate, a weak base. This protonation leads to the formation of carbonic acid (H₂CO₃):
CaCO₃ + 2H⁺ → Ca²⁺ + H₂CO₃
This step involves the transfer of a proton from the hydronium ion (H₃O⁺), formed by the dissociation of HCl in water, to the carbonate ion (CO₃²⁻).
Step 2: Decomposition of Carbonic Acid
Carbonic acid is an unstable compound and readily decomposes into water and carbon dioxide:
H₂CO₃ → H₂O + CO₂
This decomposition releases carbon dioxide gas, which is often observed as effervescence or bubbling during the reaction. The overall reaction is exothermic, meaning it releases heat.
Practical Applications
The reaction between calcium carbonate and hydrochloric acid has numerous practical applications across various fields:
1. Digestion and Antacid Action:
In the human digestive system, stomach acid (primarily HCl) helps break down food. Antacids, often containing calcium carbonate, neutralize excess stomach acid, relieving heartburn and indigestion. The reaction neutralizes the acidity, reducing discomfort.
2. Industrial Cleaning:
Calcium carbonate deposits, like limescale, are often found in pipes and industrial equipment. Hydrochloric acid is used to remove these deposits, effectively cleaning the surfaces. The reaction dissolves the calcium carbonate, leaving behind calcium chloride and water.
3. Laboratory Applications:
The reaction is used in various laboratory experiments to demonstrate acid-base reactions, gas production, and stoichiometric calculations. It’s a simple and effective way to illustrate chemical principles.
4. Geological Processes:
This reaction plays a significant role in the weathering of limestone and marble (both primarily composed of calcium carbonate). Acid rain, containing dissolved acids like sulfuric and nitric acids, reacts with these rocks, leading to their gradual dissolution and erosion. The process contributes to the formation of caves and other geological features.
5. Cement Production:
The reaction is relevant in the production of cement. Calcium carbonate (limestone) is a key ingredient in the manufacturing process, interacting with other components to form the cement.
6. Analysis of Calcium Carbonate Content:
The reaction can be utilized to determine the amount of calcium carbonate in a sample. By carefully measuring the volume of hydrochloric acid required to completely react with a known mass of the sample, the amount of calcium carbonate can be calculated using stoichiometry. This is a common technique in analytical chemistry.
Safety Considerations
While this reaction is relatively straightforward, certain safety precautions must be followed:
- Hydrochloric Acid: Hydrochloric acid is corrosive and can cause burns to the skin and eyes. Always wear appropriate personal protective equipment (PPE), including gloves, eye protection, and a lab coat.
- Carbon Dioxide: The released carbon dioxide can displace oxygen in a confined space, creating a risk of asphyxiation. Ensure adequate ventilation during the reaction.
- Exothermic Reaction: The reaction is exothermic, meaning it produces heat. This heat can be significant, particularly with larger amounts of reactants. Conduct the reaction in a controlled environment to prevent accidental burns or damage.
- Waste Disposal: The reaction produces calcium chloride, a soluble salt. Proper disposal of the waste is crucial to prevent environmental contamination.
Further Exploration and Related Reactions
This reaction serves as a foundation for understanding similar reactions involving other carbonates and acids. For instance, the reaction between sodium carbonate (Na₂CO₃) and hydrochloric acid follows a similar mechanism, producing sodium chloride, water, and carbon dioxide. Other carbonates, such as magnesium carbonate (MgCO₃), react with HCl in a comparable manner.
Exploring the kinetics of this reaction – the rate at which it proceeds – can reveal valuable insights into reaction mechanisms and influencing factors. The reaction rate depends on factors such as concentration of reactants, temperature, and the presence of catalysts.
The reaction’s equilibrium constant can be determined experimentally, providing quantitative information about the relative amounts of reactants and products at equilibrium. This equilibrium constant offers further understanding of the reaction's spontaneity and the position of equilibrium.
Finally, the reaction's thermodynamics – the heat change associated with the reaction – provides information about the energy involved in the process. The enthalpy change (ΔH) can be measured experimentally to quantify the amount of heat released or absorbed during the reaction.
Conclusion
The reaction between calcium carbonate and hydrochloric acid is a fundamental chemical process with broad implications across various fields. Understanding its balanced equation, underlying mechanism, and practical applications is essential for students and professionals in chemistry, geology, and related disciplines. Always remember to prioritize safety when handling these chemicals and to dispose of waste materials responsibly. Further exploration into the kinetics, equilibrium, and thermodynamics of the reaction offers a deeper understanding of its chemical behavior and its impact on the world around us. The reaction remains a key example of an acid-base reaction with practical significance and theoretical interest.
Latest Posts
Latest Posts
-
All Of The Following Are Communicable Diseases Except
Apr 09, 2025
-
Through Which Of The Following Media Can Sound Waves Travel
Apr 09, 2025
-
Study Of Animal Behaviour Is Called
Apr 09, 2025
-
What Is The Final Product Of The Calvin Cycle
Apr 09, 2025
-
Write A Letter For Increment Of Salary
Apr 09, 2025
Related Post
Thank you for visiting our website which covers about Calcium Carbonate And Hydrochloric Acid Balanced Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.