Balanced Equation Of Naoh And H2so4
News Leon
Mar 15, 2025 · 5 min read
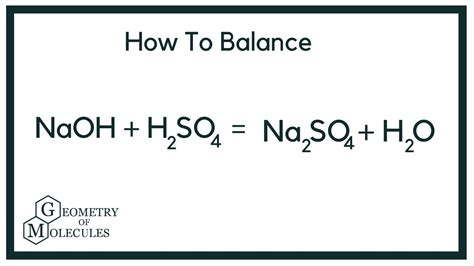
Table of Contents
The Balanced Equation of NaOH and H₂SO₄: A Deep Dive into Acid-Base Reactions
The reaction between sodium hydroxide (NaOH) and sulfuric acid (H₂SO₄) is a classic example of an acid-base neutralization reaction. Understanding this reaction, including its balanced equation, stoichiometry, and practical applications, is fundamental to chemistry. This comprehensive guide delves into the intricacies of this reaction, providing a detailed explanation suitable for students and enthusiasts alike.
Understanding the Reactants
Before diving into the balanced equation, let's briefly review the properties of the reactants:
Sodium Hydroxide (NaOH)
Sodium hydroxide, also known as caustic soda or lye, is a strong base. This means it readily dissociates in water to release hydroxide ions (OH⁻), significantly increasing the solution's pH. Its properties make it crucial in various industrial and household applications, from drain cleaning to soap manufacturing. It's a highly corrosive substance and requires careful handling.
Sulfuric Acid (H₂SO₄)
Sulfuric acid is a strong diprotic acid. "Diprotic" signifies it can donate two protons (H⁺ ions) per molecule during a reaction. This characteristic is key to understanding the stoichiometry of its reaction with NaOH. Like NaOH, H₂SO₄ is highly corrosive and requires careful handling. Its industrial uses are extensive, including fertilizer production, metal processing, and petroleum refining.
The Balanced Equation: A Step-by-Step Explanation
The reaction between NaOH and H₂SO₄ produces sodium sulfate (Na₂SO₄) and water (H₂O). The unbalanced equation is:
NaOH + H₂SO₄ → Na₂SO₄ + H₂O
This equation is unbalanced because the number of atoms of each element isn't equal on both sides. To balance it, we need to adjust the stoichiometric coefficients (the numbers in front of each chemical formula):
1. Balancing Sodium (Na): There's one sodium atom on the left and two on the right. To balance this, we place a 2 in front of NaOH:
2NaOH + H₂SO₄ → Na₂SO₄ + H₂O
2. Balancing Sulfate (SO₄): The sulfate ion (SO₄²⁻) is a polyatomic ion that remains intact throughout the reaction. We have one sulfate ion on each side, so it's already balanced.
3. Balancing Hydrogen (H): There are four hydrogen atoms on the left (two from 2NaOH and two from H₂SO₄) and only two on the right. To balance, we place a 2 in front of H₂O:
2NaOH + H₂SO₄ → Na₂SO₄ + 2H₂O
4. Balancing Oxygen (O): Finally, let's check the oxygen atoms. We have six oxygen atoms on both sides (two from 2NaOH, four from H₂SO₄, and four from 2H₂O and Na₂SO₄). The equation is now balanced.
Therefore, the fully balanced equation is:
2NaOH + H₂SO₄ → Na₂SO₄ + 2H₂O
Stoichiometry and Mole Ratios
The balanced equation provides crucial information about the stoichiometry of the reaction. The coefficients indicate the mole ratios of the reactants and products:
- 2 moles of NaOH react with 1 mole of H₂SO₄. This means that for every two moles of sodium hydroxide used, one mole of sulfuric acid is required for complete neutralization.
- 1 mole of Na₂SO₄ and 2 moles of H₂O are produced. The reaction yields one mole of sodium sulfate and two moles of water for every mole of sulfuric acid consumed.
Understanding these mole ratios is essential for performing stoichiometric calculations, such as determining the amount of product formed from a given amount of reactant or determining the limiting reactant in a reaction mixture.
Titration: A Practical Application
One of the most important applications of the NaOH and H₂SO₄ reaction is in acid-base titrations. Titration is a laboratory technique used to determine the concentration of an unknown solution (analyte) using a solution of known concentration (titrant).
In this case, we can use a standardized solution of NaOH (known concentration) to titrate an unknown solution of H₂SO₄. By carefully measuring the volume of NaOH required to neutralize a known volume of H₂SO₄, we can calculate the concentration of the sulfuric acid using the balanced equation and the mole ratios. The endpoint of the titration is usually determined using a pH indicator, which changes color near the equivalence point (where the moles of acid equal the moles of base).
Types of Reactions Involved
The reaction between NaOH and H₂SO₄ is fundamentally a neutralization reaction. This is a specific type of double displacement reaction, where the cations and anions of the reactants switch partners to form new compounds. More specifically, it's an acid-base neutralization reaction, resulting in the formation of a salt (Na₂SO₄) and water (H₂O).
Factors Affecting the Reaction
Several factors can influence the rate and extent of the reaction between NaOH and H₂SO₄:
- Concentration of reactants: Higher concentrations of reactants generally lead to a faster reaction rate.
- Temperature: Increasing the temperature usually increases the reaction rate.
- Presence of catalysts: While not common in this specific reaction, catalysts could potentially accelerate the reaction rate.
- Mixing: Proper mixing ensures the reactants come into contact, promoting a faster and more complete reaction.
Safety Precautions
Both NaOH and H₂SO₄ are highly corrosive substances. Always wear appropriate personal protective equipment (PPE), including safety goggles, gloves, and a lab coat when handling these chemicals. Work in a well-ventilated area and follow proper laboratory safety procedures. In case of skin or eye contact, immediately flush the affected area with copious amounts of water and seek medical attention if necessary.
Beyond the Basics: Exploring Further Applications
The sodium sulfate (Na₂SO₄) produced in this reaction has numerous applications:
- Detergents: Sodium sulfate is used as a filler in detergents to control viscosity and improve its performance.
- Paper Manufacturing: It acts as a filler and sizing agent in paper production.
- Textiles: It finds use in dyeing and printing textiles.
- Medicine: It's used as a laxative and also in some contrast media for medical imaging.
Understanding the balanced equation of NaOH and H₂SO₄ opens doors to a deeper understanding of acid-base chemistry and its vast applications across various fields. The reaction is fundamental to many industrial processes and analytical techniques. The stoichiometry and principles discussed here are transferable to many other acid-base reactions. Remember to always prioritize safety when handling these corrosive chemicals.
Latest Posts
Latest Posts
-
Why Are Producers So Important To An Ecosystem
Mar 15, 2025
-
Difference Between Interest Groups And Political Parties
Mar 15, 2025
-
The Division Of The Cell Nucleus Is Called
Mar 15, 2025
-
The Summer Of The White Beautiful Horse
Mar 15, 2025
-
Which Phase Of Cell Cycle Is Longest
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Balanced Equation Of Naoh And H2so4 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
