Balanced Equation For Hydrochloric Acid And Calcium Carbonate
News Leon
Apr 06, 2025 · 5 min read
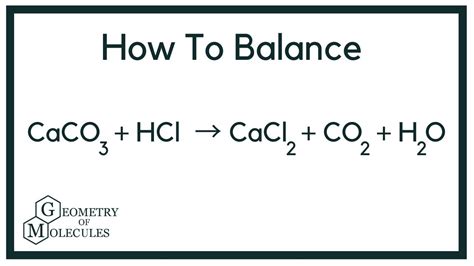
Table of Contents
The Balanced Equation for Hydrochloric Acid and Calcium Carbonate: A Deep Dive
The reaction between hydrochloric acid (HCl) and calcium carbonate (CaCO₃) is a classic example of an acid-base reaction, specifically a neutralization reaction. Understanding this reaction, from its balanced equation to its applications and implications, is crucial in various fields, including chemistry, geology, and even medicine. This comprehensive article will delve into every aspect of this reaction, exploring the balanced equation, the underlying mechanisms, practical applications, and safety considerations.
The Balanced Chemical Equation
The reaction between hydrochloric acid and calcium carbonate produces calcium chloride, carbon dioxide, and water. The unbalanced equation looks like this:
HCl + CaCO₃ → CaCl₂ + CO₂ + H₂O
This equation, however, isn't balanced. A balanced chemical equation ensures that the number of atoms of each element is equal on both the reactant and product sides. To achieve this balance, we adjust the coefficients in front of the chemical formulas:
2HCl + CaCO₃ → CaCl₂ + CO₂ + H₂O
Now, let's verify the balance:
- Hydrogen (H): 2 atoms on the reactant side and 2 atoms on the product side.
- Chlorine (Cl): 2 atoms on the reactant side and 2 atoms on the product side.
- Calcium (Ca): 1 atom on the reactant side and 1 atom on the product side.
- Carbon (C): 1 atom on the reactant side and 1 atom on the product side.
- Oxygen (O): 3 atoms on the reactant side and 3 atoms on the product side.
The equation is now balanced, meaning the Law of Conservation of Mass is satisfied. This law dictates that matter cannot be created or destroyed in a chemical reaction; only rearranged.
Understanding the Reaction Mechanism
The reaction between hydrochloric acid and calcium carbonate is a two-step process:
Step 1: Acid-Base Neutralization
Hydrochloric acid, a strong acid, donates a proton (H⁺) to calcium carbonate, a weak base. This protonation leads to the formation of carbonic acid (H₂CO₃):
2HCl + CaCO₃ → Ca²⁺ + 2Cl⁻ + H₂CO₃
This step represents a typical acid-base neutralization reaction. The calcium carbonate acts as a base, accepting the protons from the hydrochloric acid. The resulting ions, calcium (Ca²⁺) and chloride (Cl⁻), remain in solution.
Step 2: Decomposition of Carbonic Acid
Carbonic acid (H₂CO₃) is unstable and readily decomposes into carbon dioxide (CO₂) and water (H₂O):
H₂CO₃ → CO₂ + H₂O
This decomposition is a spontaneous process, releasing carbon dioxide gas, which is often observed as effervescence (bubbling) during the reaction. The water remains in solution.
Practical Applications
The reaction between hydrochloric acid and calcium carbonate has numerous practical applications across various disciplines:
1. Digestion of Antacids
Many antacids contain calcium carbonate as an active ingredient. When these antacids are ingested, they react with the excess hydrochloric acid in the stomach, neutralizing the acid and relieving heartburn or indigestion. The reaction produces carbon dioxide gas, which is often responsible for the belching associated with antacid use.
2. Cleaning of Limestone and Marble
Limestone and marble are primarily composed of calcium carbonate. Hydrochloric acid can be used to clean these materials, removing dirt and grime by dissolving the calcium carbonate. However, this cleaning method should be used cautiously as it can etch or damage the surface if not properly controlled.
3. Laboratory Applications
In the laboratory setting, this reaction is used for various purposes:
- Quantitative analysis: The reaction can be used to determine the concentration of hydrochloric acid or calcium carbonate through titration.
- Preparation of calcium chloride: Calcium chloride, a common salt, can be produced by this reaction.
4. Geological Processes
This reaction plays a significant role in geological processes, such as:
- Cave formation: The dissolution of limestone by slightly acidic rainwater (containing carbonic acid) over millions of years leads to the formation of caves. The reaction with hydrochloric acid, though less prevalent naturally, shares a similar chemical mechanism.
- Weathering of rocks: Calcium carbonate in rocks is gradually weathered and dissolved by acidic substances, including those derived from the decomposition of organic matter, contributing to soil formation.
Safety Precautions
When working with hydrochloric acid and calcium carbonate, several safety precautions should be taken:
- Eye protection: Always wear safety goggles or a face shield to protect your eyes from splashes.
- Gloves: Wear chemical-resistant gloves to prevent skin contact with the acid.
- Ventilation: Conduct the reaction in a well-ventilated area to avoid inhaling carbon dioxide gas.
- Acid handling: Handle hydrochloric acid carefully, avoiding spills and splashes.
- Disposal: Dispose of the reaction waste properly according to local regulations. Never pour acid down the drain without proper neutralization.
Further Considerations
The rate of the reaction between hydrochloric acid and calcium carbonate can be influenced by several factors:
- Concentration of the acid: A higher concentration of hydrochloric acid will generally lead to a faster reaction rate.
- Surface area of calcium carbonate: A larger surface area of calcium carbonate (e.g., powdered form) will result in a faster reaction rate due to increased contact between the reactants.
- Temperature: Increasing the temperature will generally increase the reaction rate.
The reaction stoichiometry, as defined by the balanced equation, provides the quantitative relationship between the reactants and products. This allows for precise calculations of the amounts of reactants needed to produce a specific amount of product, or vice-versa. Understanding this stoichiometry is vital for any practical application of this reaction, whether in industrial processes, laboratory experiments, or everyday life.
Conclusion
The reaction between hydrochloric acid and calcium carbonate is a fundamental chemical reaction with far-reaching implications. Understanding the balanced equation, the reaction mechanism, and the associated safety precautions is crucial for anyone working with these chemicals. Its applications span a wide range of fields, from everyday digestion to geological processes and industrial applications. By appreciating the significance and nuances of this seemingly simple reaction, we gain a deeper understanding of the fundamental principles of chemistry and their relevance in the world around us. The reaction's versatility underscores the importance of mastering basic chemical principles in solving real-world problems and further scientific exploration. Continued research and innovation in related fields will undoubtedly uncover even more applications and refinements in utilizing this ubiquitous chemical process.
Latest Posts
Latest Posts
-
The Quotient Of 3 And A Number
Apr 06, 2025
-
Where Is The Rhythmicity Center For Respiration
Apr 06, 2025
-
Which Two Elements Most Likely Have The Most Similar Properties
Apr 06, 2025
-
Why Doesnt Hydrogen Have A Neutron
Apr 06, 2025
-
A Number Is Divisible By 2 If
Apr 06, 2025
Related Post
Thank you for visiting our website which covers about Balanced Equation For Hydrochloric Acid And Calcium Carbonate . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
