Atomic Mass Is Determined By The Number Of
News Leon
Apr 06, 2025 · 6 min read
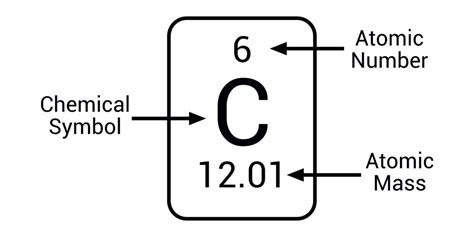
Table of Contents
Atomic Mass: Determined by the Number of Protons and Neutrons
Atomic mass, a fundamental concept in chemistry and physics, dictates the mass of an atom. It's not simply a random number; it's directly tied to the building blocks within the atom's nucleus. Understanding atomic mass is crucial for comprehending chemical reactions, isotopic variations, and the very nature of matter itself. This article will delve deep into the relationship between atomic mass and the number of protons and neutrons within an atom's nucleus.
The Nucleus: The Heart of the Atom
At the heart of every atom lies the nucleus, a dense region containing two types of subatomic particles: protons and neutrons. These particles are collectively referred to as nucleons.
-
Protons: Positively charged particles that determine the element's identity. The number of protons defines the atomic number (Z) and uniquely identifies an element on the periodic table. For example, all atoms with one proton are hydrogen, those with six are carbon, and those with 92 are uranium.
-
Neutrons: Neutral particles (no charge) that contribute significantly to the atom's mass but not its chemical properties. The number of neutrons can vary within the same element, leading to the existence of isotopes.
Isotopes: Variations on a Theme
Isotopes are atoms of the same element (same number of protons) that possess different numbers of neutrons. This difference in neutron count leads to variations in the atom's mass. While they have the same chemical behavior due to their identical proton number, their physical properties, such as density and radioactivity, can differ significantly.
Example: Carbon-12 (¹²C) has 6 protons and 6 neutrons, while Carbon-14 (¹⁴C) has 6 protons and 8 neutrons. Both are carbon atoms, but their masses differ due to the extra neutrons in ¹⁴C. Carbon-14 is also radioactive, unlike the stable Carbon-12.
Atomic Mass Unit (amu): Measuring the Mass of Atoms
Measuring the mass of individual atoms is a challenge. Instead, scientists use the atomic mass unit (amu), also known as the dalton (Da). One amu is defined as one-twelfth the mass of a single carbon-12 atom. This means a single carbon-12 atom has a mass of exactly 12 amu.
The mass of a proton is approximately 1 amu, and the mass of a neutron is also approximately 1 amu. Electrons, while contributing to the atom's charge, have a negligible mass compared to protons and neutrons and are often ignored when calculating atomic mass.
Calculating Atomic Mass: The Role of Isotopes
The atomic mass listed on the periodic table for an element isn't the mass of a single atom but rather a weighted average of the masses of all naturally occurring isotopes of that element. This weighted average takes into account the abundance of each isotope in nature.
Formula for calculating the atomic mass of an element:
Atomic mass = (mass of isotope 1 × % abundance of isotope 1) + (mass of isotope 2 × % abundance of isotope 2) + ...
Example Calculation: Chlorine has two main isotopes: chlorine-35 (³⁵Cl) and chlorine-37 (³⁷Cl). ³⁵Cl has an abundance of 75.77% and a mass of approximately 35 amu, while ³⁷Cl has an abundance of 24.23% and a mass of approximately 37 amu.
Atomic mass of Chlorine = (35 amu × 0.7577) + (37 amu × 0.2423) ≈ 35.45 amu
This calculated atomic mass of approximately 35.45 amu is the value found on the periodic table for chlorine.
Factors Affecting Atomic Mass
Several factors influence the atomic mass of an element:
-
Number of Protons and Neutrons: The primary determinant of atomic mass, as already discussed. More nucleons mean a higher atomic mass.
-
Isotopic Abundance: The relative abundance of each isotope in a naturally occurring sample significantly impacts the weighted average atomic mass. A higher abundance of a heavier isotope leads to a higher overall atomic mass for the element.
-
Nuclear Binding Energy: The energy required to hold the nucleons together within the nucleus also contributes, albeit minimally, to the atomic mass. The mass of the nucleus is slightly less than the sum of the masses of its individual protons and neutrons due to the conversion of mass into energy according to Einstein's famous equation, E=mc². This mass defect is a tiny fraction of the overall atomic mass but is a significant concept in nuclear physics.
Applications of Atomic Mass
Understanding atomic mass has wide-ranging applications across various scientific fields:
-
Stoichiometry: Atomic mass is crucial for calculating the amounts of reactants and products in chemical reactions. It allows chemists to determine the precise amounts of substances needed for a reaction to proceed efficiently.
-
Nuclear Chemistry: Atomic mass is fundamental to understanding nuclear reactions, such as nuclear fission and fusion. Precise knowledge of atomic mass allows scientists to predict the energy released during these reactions.
-
Mass Spectrometry: This analytical technique uses the mass-to-charge ratio of ions to identify and quantify different isotopes within a sample. It relies heavily on the precise atomic masses of individual isotopes.
-
Geochemistry: Isotopic ratios of elements in geological samples are used to determine the age of rocks and minerals. This technique, called radiometric dating, depends on the understanding of radioactive decay and the known half-lives of isotopes, which are directly linked to atomic mass and nuclear stability.
-
Medical Imaging and Treatment: Radioactive isotopes, with specific atomic masses, are utilized in various medical applications, including PET scans (positron emission tomography) and radiation therapy for cancer treatment. The selection of isotopes is based on their radioactive properties, decay rates and the atomic mass influence the properties.
Beyond the Basics: Nuclear Stability and Atomic Mass
The number of neutrons relative to the number of protons in an atom's nucleus plays a critical role in determining its stability. Atoms with a stable neutron-to-proton ratio tend to be non-radioactive, while those with an unstable ratio are often radioactive, meaning their nuclei decay over time to achieve a more stable configuration. This decay can involve the emission of alpha particles, beta particles, or gamma rays. The atomic mass of the radioactive isotopes is key to understanding the type and energy of radiation emitted during decay.
The "valley of stability" is a graphical representation of stable isotopes on a plot of the number of neutrons versus the number of protons. Isotopes falling outside this valley are generally radioactive and undergo nuclear decay to reach a more stable state. The atomic mass gives a clear indicator of location of the isotope. Understanding the relationship between atomic mass, neutron-proton ratio, and nuclear stability is vital in nuclear physics and chemistry.
Conclusion: Atomic Mass - A Cornerstone of Chemistry and Physics
Atomic mass, determined by the combined number of protons and neutrons in an atom's nucleus, is a fundamental concept that underpins many areas of science. Its understanding is crucial for comprehending chemical reactions, isotopic variations, and nuclear processes. From calculating the quantities in stoichiometric calculations to determining the age of geological samples and developing medical imaging techniques, atomic mass plays a vital role. The weighted average atomic mass, as presented on the periodic table, reflects the isotopic abundance in nature and serves as a cornerstone for various scientific applications. The relationship between atomic mass, nuclear stability, and radioactive decay is a critical area of study, continually advancing our knowledge of matter and energy at the subatomic level. Continued exploration of atomic mass will undoubtedly lead to further advancements in our understanding of the universe.
Latest Posts
Latest Posts
-
Why Are Petals Usually Brightly Colored
Apr 08, 2025
-
Correct The Following Sentence And Rewrite Them
Apr 08, 2025
-
The Ph Of 0 1 Molar Ammonia Is Approximately
Apr 08, 2025
-
Is Na A Metal Nonmetal Or Metalloid
Apr 08, 2025
-
What Subatomic Particle Is The Heaviest
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about Atomic Mass Is Determined By The Number Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.