Are Glucose And Fructose Structural Isomers
News Leon
Apr 06, 2025 · 5 min read
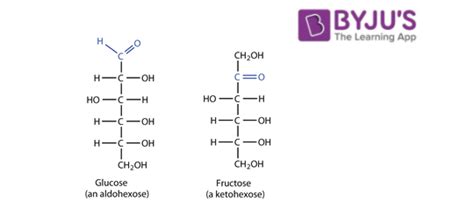
Table of Contents
Are Glucose and Fructose Structural Isomers? A Deep Dive into Monosaccharide Structures
The question of whether glucose and fructose are structural isomers is a fundamental concept in organic chemistry, particularly within the realm of carbohydrate chemistry. Understanding their relationship requires a thorough examination of their molecular structures, chemical properties, and biological functions. While they share the same molecular formula (C₆H₁₂O₆), their distinct structural arrangements lead to significant differences in their properties and roles in biological systems. This article will delve deep into the intricacies of glucose and fructose, clarifying their isomeric relationship and exploring the implications of their structural variations.
Understanding Isomerism
Before diving into the specifics of glucose and fructose, let's establish a clear understanding of isomerism. Isomers are molecules that share the same molecular formula but differ in the arrangement of their atoms. This seemingly subtle difference can lead to vastly different chemical and physical properties. There are several types of isomerism, including:
1. Structural Isomerism (Constitutional Isomerism):
Structural isomers have the same molecular formula but differ in the way their atoms are connected. This means the atoms are bonded in a different order or sequence. This is the key type of isomerism we'll be focusing on when comparing glucose and fructose.
2. Stereoisomerism:
Stereoisomers have the same molecular formula and the same connectivity of atoms, but they differ in the spatial arrangement of their atoms. This includes geometric isomers (cis-trans isomers) and optical isomers (enantiomers and diastereomers). While glucose and fructose exhibit stereoisomerism, it's their structural differences that are most pertinent to the question at hand.
Glucose: The Primary Energy Source
Glucose is an aldohexose, meaning it's a six-carbon sugar (hexose) containing an aldehyde functional group (-CHO) at one end of the carbon chain. Its linear structure can be represented as follows:
CHO - (CHOH)₄ - CH₂OH
However, glucose predominantly exists in a cyclic form, forming a six-membered ring structure (pyranose) through an intramolecular reaction between the aldehyde group and a hydroxyl group on carbon 5. This cyclization leads to the formation of two anomers, α-glucose and β-glucose, which differ in the orientation of the hydroxyl group at the anomeric carbon (carbon 1). The cyclic structures of α-glucose and β-glucose are crucial for their roles in various biological processes, including energy storage and metabolism.
Fructose: The Fruit Sugar
Fructose, on the other hand, is a ketohexose, meaning it's a six-carbon sugar with a ketone functional group (=C=O) located on carbon 2. Its linear form is:
CH₂OH - (CHOH)₃ - C(=O) - CH₂OH
Similar to glucose, fructose also predominantly exists in cyclic forms. However, fructose commonly forms a five-membered ring structure (furanose) through a reaction between the ketone group and a hydroxyl group on carbon 5. This results in α-fructose and β-fructose anomers, each with unique properties.
Glucose and Fructose: Structural Differences and Isomeric Relationship
The crucial point to consider is that glucose and fructose are indeed structural isomers. They have the same molecular formula (C₆H₁₂O₆), but their atoms are connected differently. The placement of the carbonyl group (aldehyde in glucose, ketone in fructose) is the primary structural difference. This seemingly small difference has profound consequences on their chemical reactivity, physical properties, and biological roles.
Detailed Comparison of Structures:
-
Carbonyl Group Location: The most significant difference lies in the location of the carbonyl group. Glucose has an aldehyde group at carbon 1, while fructose has a ketone group at carbon 2. This alters the reactivity and the overall structure of the molecule.
-
Cyclic Structures: Although both form cyclic structures, the ring sizes differ. Glucose predominantly exists as a six-membered pyranose ring, while fructose commonly forms a five-membered furanose ring. This difference in ring size significantly impacts their steric properties and interactions with enzymes.
-
Hydroxyl Group Orientation: The orientation of hydroxyl groups on different carbons further contributes to the structural differences. While subtle, these differences directly affect how these sugars interact with enzymes and other molecules in biological systems.
-
Conformations: The different ring sizes and hydroxyl group orientations lead to a variety of different conformations for both glucose and fructose. These conformational differences affect how these sugars interact with their biological environment.
Biological Implications of Structural Differences
The structural differences between glucose and fructose have significant consequences for their biological roles:
-
Metabolism: Glucose is the primary source of energy for most organisms. It's directly metabolized through glycolysis, a fundamental metabolic pathway. Fructose, however, requires a different metabolic pathway, initially involving fructokinase and eventually entering the glycolytic pathway at a different point.
-
Sweetness: Fructose is significantly sweeter than glucose. This is attributed to its structural differences and how it interacts with the sweetness receptors on the tongue.
-
Energy Storage: Glucose is stored in the body as glycogen, a branched polysaccharide. Fructose, while also capable of being converted into energy, isn't stored in the same manner.
-
Enzyme Specificity: Enzymes involved in carbohydrate metabolism exhibit high specificity. Different enzymes act on glucose and fructose, reflecting their structural differences.
Beyond the Basics: Advanced Concepts
The isomeric relationship between glucose and fructose extends beyond simple structural isomerism. They are also stereoisomers. Specifically, they are diastereomers, meaning they are stereoisomers that are not mirror images of each other. Understanding this complete picture requires delving into the intricacies of chiral centers and Fischer projections, advanced topics beyond the scope of a simple explanation. However, appreciating the multifaceted nature of their isomerism enriches the understanding of their distinct properties and functions.
Conclusion
Glucose and fructose are indeed structural isomers, sharing the same molecular formula but differing in the connectivity of their atoms, primarily in the location of the carbonyl group. This fundamental structural difference leads to significant variations in their chemical properties, metabolic pathways, sweetness perception, and overall biological roles. Understanding this isomeric relationship is crucial for comprehending the fundamental processes of carbohydrate metabolism and the diverse functions of these essential monosaccharides in biological systems. Their differences, though seemingly subtle at the molecular level, have profound and far-reaching consequences within the complex world of biochemistry. The seemingly simple question of whether they are isomers unveils a rich tapestry of chemical and biological intricacy.
Latest Posts
Latest Posts
-
Is Neon Metal Nonmetal Or Metalloid
Apr 09, 2025
-
Why Is A Circle Not A Polygon
Apr 09, 2025
-
Center Of Mass Of Quarter Circle
Apr 09, 2025
-
Rate Constant Units For Third Order Reaction
Apr 09, 2025
-
What Protects And Supports The Cell
Apr 09, 2025
Related Post
Thank you for visiting our website which covers about Are Glucose And Fructose Structural Isomers . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.