An Element Is Defined By The Number Of
News Leon
Mar 18, 2025 · 7 min read
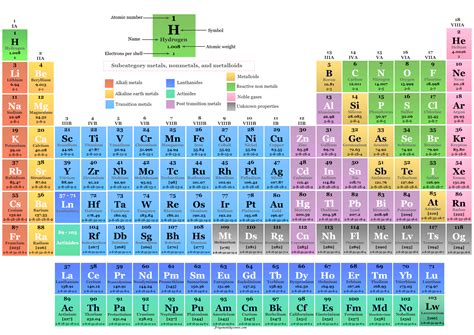
Table of Contents
An Element is Defined by the Number of Protons: A Deep Dive into Atomic Structure and Properties
The fundamental building blocks of matter, elements, are not defined by their mass, their number of neutrons, or even their number of electrons. Instead, an element is defined by the number of protons in its nucleus. This seemingly simple statement underpins the entire field of chemistry and is crucial to understanding the properties and behavior of all substances. This article will delve deep into this fundamental concept, exploring its implications and connections to other atomic properties.
Understanding Atomic Structure: The Nucleus and its Inhabitants
Before we delve into the defining role of protons, let's review the basic structure of an atom. Atoms are composed of three subatomic particles:
- Protons: Positively charged particles residing within the atom's nucleus.
- Neutrons: Neutrally charged particles also found within the nucleus.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels.
The nucleus, a tiny, dense core at the atom's center, contains both protons and neutrons. The electrons, comparatively much lighter, occupy the space surrounding the nucleus. The arrangement of these particles dictates an atom's chemical behavior and physical properties.
The Atomic Number: The Unique Identifier of an Element
The number of protons in an atom's nucleus is its atomic number. This number is unique to each element and is what truly defines it. For instance, all hydrogen atoms possess one proton (atomic number 1), all helium atoms have two protons (atomic number 2), and all oxygen atoms have eight protons (atomic number 8). No two elements share the same atomic number. This is the fundamental principle that organizes the periodic table of elements, a crucial tool in chemistry and physics.
The atomic number is not just a convenient label; it dictates a vast array of an element's characteristics. It determines:
- Chemical properties: How an element interacts with other elements to form compounds. This is primarily determined by the number of electrons, which is directly related to the number of protons.
- Physical properties: Characteristics such as melting point, boiling point, density, and conductivity. These properties are influenced by the interplay of protons, neutrons, and electrons.
- Placement on the periodic table: Elements are arranged based on their atomic numbers, reflecting their periodic trends in properties.
Isotopes: Variations in Neutron Number
While the number of protons defines an element, the number of neutrons can vary. Atoms of the same element with different numbers of neutrons are called isotopes. Isotopes of an element share the same atomic number (number of protons) but differ in their mass number (the sum of protons and neutrons).
For example, carbon has three naturally occurring isotopes: carbon-12 (6 protons, 6 neutrons), carbon-13 (6 protons, 7 neutrons), and carbon-14 (6 protons, 8 neutrons). All are carbon because they all possess six protons, but they differ slightly in their mass and properties due to the varying number of neutrons. Some isotopes are stable, while others are radioactive, meaning their nuclei decay over time.
The presence of isotopes explains why the atomic mass (average mass of an element's atoms, taking into account the abundance of its isotopes) is not a whole number for many elements. The atomic mass reflects the weighted average of the masses of all naturally occurring isotopes of that element.
Electron Configuration and Chemical Behavior
The number of protons dictates the number of electrons in a neutral atom (equal numbers of protons and electrons). The arrangement of these electrons in various energy levels or shells determines the element's chemical behavior. Electrons in the outermost shell, called valence electrons, are particularly crucial in forming chemical bonds with other atoms.
The periodic table's organization reflects the repeating patterns in electron configurations. Elements in the same group (column) have similar valence electron arrangements, leading to similar chemical properties. This periodic recurrence of properties is a direct consequence of the underlying principle that an element is defined by its atomic number – the number of protons.
The Role of Protons in Nuclear Reactions
Protons play a vital role in nuclear reactions, processes that involve changes in an atom's nucleus. Nuclear fission, for instance, involves the splitting of a heavy atomic nucleus into lighter nuclei, often releasing tremendous amounts of energy. The number of protons in the resulting nuclei determines the elements formed during fission.
Nuclear fusion, on the other hand, involves the combining of lighter atomic nuclei to form a heavier nucleus, also releasing significant energy. Again, the number of protons determines the identity of the newly formed element. These nuclear reactions are fundamental to processes like stellar nucleosynthesis, the creation of elements within stars.
Protons and the Periodic Table: A Deeper Look
The periodic table is a testament to the significance of the atomic number. It's a visual representation of the elements, organized by their atomic numbers. The arrangement reflects the periodic recurrence of chemical and physical properties, arising from the regular pattern of electron configurations.
The table's rows (periods) represent the filling of electron shells, while the columns (groups) represent elements with similar valence electron configurations and, consequently, similar chemical behavior. The alkali metals (Group 1) all have one valence electron, leading to their high reactivity. The noble gases (Group 18), with full valence shells, are remarkably inert. These trends are directly linked to the number of protons and resulting electron configurations.
Beyond the Basics: Exploring Isobaric and Isotonic Relationships
While the atomic number uniquely defines an element, understanding other relationships between isotopes and different elements adds further depth to the concept.
- Isobars: Atoms with the same mass number (protons + neutrons) but different atomic numbers (different elements). For example, ¹⁴C (carbon-14) and ¹⁴N (nitrogen-14) are isobars, both having a mass number of 14 but different numbers of protons.
- Isotones: Atoms with the same number of neutrons but different atomic numbers (different elements) and different mass numbers. For example, ¹⁴C (carbon-14) and ¹⁵N (nitrogen-15) are isotones, both having 8 neutrons but differing in their numbers of protons and hence their mass numbers.
Understanding these relationships helps in comprehending nuclear stability, radioactive decay, and the abundance of different isotopes in nature.
Applications and Significance
The understanding that an element is defined by the number of protons has far-reaching implications across numerous scientific fields:
- Chemistry: It forms the foundation of chemical bonding, reactivity, and the synthesis of new compounds.
- Nuclear Physics: It is central to understanding nuclear reactions, radioactive decay, and the creation of new elements.
- Materials Science: It helps in designing materials with specific properties by carefully selecting elements based on their atomic numbers and resulting characteristics.
- Medicine: Radioactive isotopes, with their unique number of neutrons, are extensively used in medical diagnostics and treatment.
- Astronomy and Astrophysics: The abundance of elements in stars and the universe provides insights into the processes of stellar nucleosynthesis and the evolution of the cosmos.
Conclusion: The Defining Role of Protons
In conclusion, the statement "an element is defined by the number of protons" is not merely a scientific fact; it is the cornerstone of our understanding of matter and its behavior. This seemingly simple concept underpins the organization of the periodic table, drives chemical reactions, and dictates the properties of all substances. Its importance extends far beyond the realm of basic chemistry, influencing various scientific disciplines and technologies. The atomic number, dictated by the number of protons, is the ultimate identifier, the fingerprint of each element, defining its place in the universe and its role in shaping the world around us. The number of protons is not just a defining feature but the very essence of what makes an element unique. Further exploration into the fascinating world of atomic structure and the properties stemming from this fundamental principle reveals the intricate and beautiful complexity of our physical world.
Latest Posts
Latest Posts
-
Word That Has Two Different Meanings
Mar 18, 2025
-
Is Soil A Homogeneous Or Heterogeneous Mixture
Mar 18, 2025
-
What Does Isinstance Do In Python
Mar 18, 2025
-
What Is The Difference Between Political Parties And Interest Groups
Mar 18, 2025
-
Electromagnetic Radiation At Its Maximum Wavelength Is
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about An Element Is Defined By The Number Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
