An Atom That Gains Or Loses An Electron Is Called
News Leon
Mar 25, 2025 · 6 min read
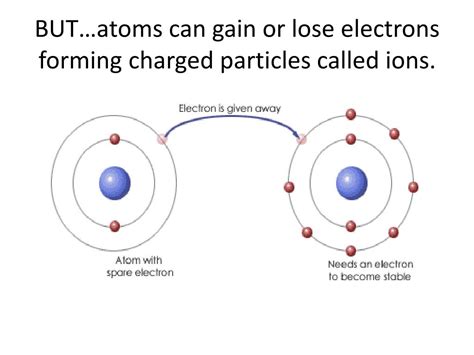
Table of Contents
An Atom That Gains or Loses an Electron is Called an Ion: A Deep Dive into Ionic Bonds and Their Significance
When an atom, the fundamental building block of matter, gains or loses an electron, it undergoes a transformation, becoming electrically charged and fundamentally altering its chemical properties. This charged atom is called an ion. Understanding ions is crucial to comprehending chemical reactions, the formation of ionic compounds, and numerous processes vital to life and technology. This comprehensive article will explore the concept of ions, delving into the processes leading to their formation, their properties, and their significant roles in various fields.
The Basics: Atoms, Electrons, and the Octet Rule
Before understanding ions, let's briefly revisit the structure of an atom. Atoms consist of a nucleus containing protons (positively charged) and neutrons (neutral), surrounded by orbiting electrons (negatively charged). The number of protons determines the atom's atomic number and its identity as a specific element. Electrons reside in energy levels or shells around the nucleus.
The octet rule is a crucial concept in understanding chemical bonding and ion formation. This rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration with eight electrons in their outermost shell (valence shell). This stable configuration resembles that of the noble gases, which are exceptionally unreactive due to their full valence shells. Exceptions to the octet rule exist, especially for elements in the transition metal series and those with low atomic numbers.
Ion Formation: Gaining and Losing Electrons
Atoms achieve a stable electron configuration primarily through two methods: gaining or losing electrons.
Cation Formation: Losing Electrons
When an atom loses one or more electrons, it becomes positively charged because the number of protons (positive charges) now exceeds the number of electrons (negative charges). This positively charged ion is called a cation. Metals, with their relatively low electronegativity (a measure of an atom's ability to attract electrons), tend to lose electrons easily to achieve a stable electron configuration.
For example, a sodium atom (Na) has one electron in its outermost shell. To achieve a stable octet, it readily loses this single electron, forming a sodium cation (Na⁺). The loss of a negatively charged electron leaves the sodium atom with a net positive charge.
Examples of Cation Formation:
- Sodium (Na) → Na⁺ + e⁻
- Magnesium (Mg) → Mg²⁺ + 2e⁻
- Aluminum (Al) → Al³⁺ + 3e⁻
Anion Formation: Gaining Electrons
Conversely, when an atom gains one or more electrons, it becomes negatively charged because the number of electrons now surpasses the number of protons. This negatively charged ion is called an anion. Nonmetals, with their higher electronegativity, tend to gain electrons to achieve a stable octet.
For example, a chlorine atom (Cl) has seven electrons in its outermost shell. To complete its octet, it readily gains one electron, forming a chloride anion (Cl⁻). The addition of a negatively charged electron gives the chlorine atom a net negative charge.
Examples of Anion Formation:
- Chlorine (Cl) + e⁻ → Cl⁻
- Oxygen (O) + 2e⁻ → O²⁻
- Nitrogen (N) + 3e⁻ → N³⁻
Properties of Ions
Ions exhibit significantly different properties compared to their neutral atoms. These differences stem from the change in their charge and electron configuration:
- Charge: The most significant difference is the presence of a net electrical charge. Cations are positively charged, while anions are negatively charged.
- Size: Cations are generally smaller than their parent atoms because they've lost electrons, reducing electron-electron repulsion. Anions, on the other hand, are typically larger than their parent atoms because they've gained electrons, increasing electron-electron repulsion.
- Reactivity: Ions are highly reactive, particularly in aqueous solutions. Their charge allows them to participate in ionic bonds and various chemical reactions.
- Physical Properties: The physical properties of ionic compounds, formed by the electrostatic attraction between cations and anions, differ significantly from those of the constituent elements. Ionic compounds often have high melting and boiling points, are brittle, and can conduct electricity when dissolved in water or melted.
Ionic Bonds: The Foundation of Ionic Compounds
The electrostatic attraction between oppositely charged ions forms a ionic bond. This strong attraction leads to the formation of ionic compounds, also known as salts. In these compounds, cations and anions are held together by a strong electrostatic force, creating a stable crystal lattice structure. The overall charge of an ionic compound is always neutral, meaning the total positive charge of the cations equals the total negative charge of the anions.
Examples of Ionic Compounds:
- Sodium chloride (NaCl): Formed from Na⁺ cations and Cl⁻ anions.
- Magnesium oxide (MgO): Formed from Mg²⁺ cations and O²⁻ anions.
- Calcium fluoride (CaF₂): Formed from Ca²⁺ cations and two F⁻ anions.
Significance of Ions in Various Fields
Ions play crucial roles in a wide range of fields:
Biology:
- Electrolyte Balance: Ions like sodium (Na⁺), potassium (K⁺), calcium (Ca²⁺), and chloride (Cl⁻) are essential electrolytes in biological systems. They maintain proper fluid balance, nerve impulse transmission, muscle contraction, and many other vital functions. Imbalances in electrolyte levels can lead to serious health problems.
- Enzyme Function: Many enzymes, biological catalysts, require specific ions as cofactors to function properly.
- DNA Structure: The negatively charged phosphate backbone of DNA relies on interactions with positively charged ions to maintain its structure and stability.
Chemistry:
- Chemical Reactions: Ions participate in a vast array of chemical reactions, forming the basis of many chemical processes in both laboratory and industrial settings.
- Electrochemistry: Ions are fundamental to electrochemistry, where chemical energy is converted into electrical energy (e.g., batteries) and vice versa (e.g., electrolysis).
Industry:
- Materials Science: Ions are crucial in the synthesis of various materials, including ceramics, glasses, and semiconductors.
- Medicine: Ions are used in various medical applications, including drug delivery systems, diagnostic imaging techniques, and treatment of electrolyte imbalances.
- Environmental Science: Monitoring ion concentrations in water bodies is essential for assessing water quality and environmental pollution.
Technology:
- Electronics: Ions are utilized in the manufacturing of electronic devices, including transistors and integrated circuits.
- Energy Storage: Ions play a key role in the development of advanced energy storage systems, such as batteries and fuel cells.
Conclusion: The Ubiquitous Importance of Ions
In conclusion, an atom that gains or loses an electron becomes an ion, a charged species that significantly alters the atom's chemical and physical properties. The formation of ions, driven by the pursuit of a stable electron configuration, leads to the formation of ionic bonds and ionic compounds, which are fundamental to a vast range of natural and technological processes. Understanding the behavior and properties of ions is paramount in various fields, including biology, chemistry, industry, and technology. From maintaining life's essential processes to powering modern electronics, the ubiquitous presence and importance of ions cannot be overstated. Further research into the behavior and manipulation of ions promises to yield even more innovative applications in the future.
Latest Posts
Latest Posts
-
What Is The Colour Of Lymph
Mar 30, 2025
-
What Is A Conductor In A Circuit
Mar 30, 2025
-
Where In The Plant Cell Does Photosynthesis Take Place
Mar 30, 2025
-
What Is The Unit Of Intensity Of Sound
Mar 30, 2025
-
Give The Iupac Names Of The Following Compounds
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about An Atom That Gains Or Loses An Electron Is Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
