Amino Acids Composed Of Carbon Hydrogen Nitrogen And Oxygen
News Leon
Mar 14, 2025 · 8 min read
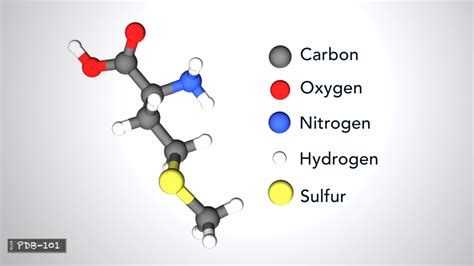
Table of Contents
Amino Acids: The Building Blocks of Life Composed of Carbon, Hydrogen, Nitrogen, and Oxygen
Amino acids are the fundamental building blocks of proteins, the workhorses of life. These organic molecules, composed primarily of carbon (C), hydrogen (H), nitrogen (N), and oxygen (O), are essential for a vast array of biological functions. Understanding their structure, properties, and roles is crucial to grasping the complexity and beauty of biological systems. This comprehensive guide delves deep into the world of amino acids, exploring their composition, classification, functions, and significance in human health and disease.
The Basic Structure of Amino Acids: A Common Foundation
All amino acids share a common core structure, a characteristic that unites this diverse group of molecules. This core consists of:
- A central carbon atom (α-carbon): This carbon atom acts as the central point of attachment for the other components.
- An amino group (-NH₂): This group is responsible for the basic properties of amino acids, as it can accept a proton (H⁺).
- A carboxyl group (-COOH): This group is responsible for the acidic properties of amino acids, as it can donate a proton (H⁺).
- A hydrogen atom (-H): This simple atom completes the basic tetrahedral structure around the α-carbon.
- A side chain (R-group): This is the variable component that distinguishes one amino acid from another. The R-group can be a simple hydrogen atom (as in glycine) or a complex structure containing various functional groups. It is the R-group that dictates the unique properties and functions of each amino acid.
The diversity of the R-groups is responsible for the incredible variety of proteins found in nature. These variations in structure lead to differences in size, charge, polarity, and reactivity, which in turn influence the protein's three-dimensional structure and its biological function.
Understanding the Importance of the R-Group
The R-group, also known as the side chain, is the unique identifier of each amino acid. Its chemical properties profoundly impact the overall characteristics and behavior of the amino acid, and subsequently the protein it forms part of. R-groups can be:
-
Nonpolar (hydrophobic): These R-groups tend to avoid water and cluster together in the interior of proteins. Examples include the aliphatic side chains of alanine, valine, leucine, and isoleucine, and the aromatic side chains of phenylalanine, tryptophan, and tyrosine.
-
Polar (hydrophilic): These R-groups interact favorably with water and are often found on the surface of proteins, interacting with the aqueous environment. Examples include serine, threonine, cysteine, asparagine, and glutamine.
-
Charged (hydrophilic): These R-groups carry a net positive or negative charge at physiological pH. Positively charged (basic) R-groups include lysine, arginine, and histidine, while negatively charged (acidic) R-groups include aspartic acid and glutamic acid.
The interaction between these different types of R-groups is crucial for protein folding and the formation of its three-dimensional structure. This structure, in turn, determines its function.
The 20 Standard Amino Acids: A Closer Look
There are 20 standard amino acids commonly found in proteins. These are the building blocks used by ribosomes during protein synthesis. Each of these amino acids has a unique R-group that imparts specific properties:
Nonpolar, Aliphatic Amino Acids
- Glycine (Gly, G): The simplest amino acid, with a hydrogen atom as its R-group. Its small size allows for greater flexibility in protein structure.
- Alanine (Ala, A): A methyl group (-CH₃) constitutes its R-group. It's a relatively simple, nonpolar amino acid.
- Valine (Val, V): A branched-chain amino acid with an isopropyl group as its R-group.
- Leucine (Leu, L): Another branched-chain amino acid, with an isobutyl group as its R-group.
- Isoleucine (Ile, I): A branched-chain amino acid with a sec-butyl group as its R-group.
Aromatic Amino Acids
- Phenylalanine (Phe, F): Contains a benzene ring as its R-group, making it hydrophobic.
- Tyrosine (Tyr, Y): Similar to phenylalanine but with a hydroxyl group (-OH) attached to the benzene ring, increasing its polarity.
- Tryptophan (Trp, W): Contains a fused benzene and pyrrole ring system in its R-group.
Polar, Uncharged Amino Acids
- Serine (Ser, S): Contains a hydroxyl group (-OH) as its R-group. This hydroxyl group can participate in hydrogen bonding.
- Threonine (Thr, T): Similar to serine, with a hydroxyl group attached to a branched carbon atom.
- Cysteine (Cys, C): Contains a thiol group (-SH) in its R-group, which can form disulfide bonds with other cysteine residues, stabilizing protein structure.
- Asparagine (Asn, N): Contains an amide group (-CONH₂) in its R-group.
- Glutamine (Gln, Q): Similar to asparagine, with a longer amide side chain.
Positively Charged (Basic) Amino Acids
- Lysine (Lys, K): Contains a long aliphatic chain ending in a primary amino group (-NH₂), giving it a strong positive charge at physiological pH.
- Arginine (Arg, R): Contains a guanidinium group in its R-group, which is strongly basic.
- Histidine (His, H): Contains an imidazole ring in its R-group, which can act as both an acid and a base, depending on the environment.
Negatively Charged (Acidic) Amino Acids
- Aspartic acid (Asp, D): Contains a carboxyl group (-COOH) in its R-group, giving it a negative charge at physiological pH.
- Glutamic acid (Glu, E): Similar to aspartic acid, with a longer carbon chain.
Amino Acid Properties and Their Impact on Protein Structure
The properties of individual amino acids, particularly their R-groups, dictate the overall characteristics and function of the proteins they form. These properties include:
-
Hydrophobicity/Hydrophilicity: The tendency of an amino acid to interact with water significantly influences protein folding. Hydrophobic amino acids tend to cluster in the protein's interior, away from water, while hydrophilic amino acids are found on the surface, interacting with the surrounding aqueous environment.
-
Charge: The charge of an amino acid's R-group at physiological pH influences its interactions with other charged molecules and contributes to the overall charge distribution within the protein. This is crucial for protein-protein interactions and enzyme-substrate binding.
-
Size and Shape: The size and shape of the R-group affect how closely amino acids can pack together in the protein's three-dimensional structure. Bulkier R-groups can restrict flexibility, while smaller R-groups allow for greater conformational freedom.
-
Ability to Form Hydrogen Bonds: Many amino acids have R-groups capable of forming hydrogen bonds, a crucial interaction in stabilizing protein structure and function.
Amino Acids and Protein Synthesis: The Ribosomal Role
The synthesis of proteins, the process of building polypeptide chains from amino acids, occurs in the ribosomes. These cellular structures read the genetic code encoded in messenger RNA (mRNA) and utilize transfer RNA (tRNA) molecules to deliver the appropriate amino acids to the growing polypeptide chain. The precise order of amino acids in a protein is dictated by the sequence of codons in the mRNA, ensuring the synthesis of functional proteins.
Essential and Non-Essential Amino Acids: Dietary Needs
Amino acids are categorized as either essential or non-essential based on the body's ability to synthesize them.
-
Essential amino acids: These cannot be synthesized by the body and must be obtained through the diet. They include: histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine.
-
Non-essential amino acids: These can be synthesized by the body and do not need to be consumed in the diet. They include: alanine, arginine, asparagine, aspartic acid, cysteine, glutamic acid, glutamine, glycine, proline, serine, and tyrosine.
A balanced diet containing sufficient amounts of essential amino acids is crucial for maintaining proper protein synthesis and overall health. Protein deficiency can lead to various health problems, including muscle wasting, impaired immune function, and stunted growth.
Amino Acids and Human Health: Beyond Protein Synthesis
The roles of amino acids extend far beyond their contribution to protein synthesis. They are involved in numerous metabolic processes and have significant implications for human health:
-
Neurotransmitter Synthesis: Several amino acids serve as precursors for neurotransmitters, chemical messengers in the nervous system. For example, tryptophan is a precursor for serotonin, a neurotransmitter involved in mood regulation, and tyrosine is a precursor for dopamine and norepinephrine, neurotransmitters involved in motivation and attention.
-
Hormone Production: Certain hormones are derived from amino acids. For instance, thyroid hormones are produced from tyrosine.
-
Immune Function: Amino acids are crucial for the synthesis of antibodies and other immune system components.
-
Energy Production: In periods of starvation or intense exercise, amino acids can be broken down to provide energy.
-
Gene Expression: Amino acids play a role in regulating gene expression, impacting various cellular processes.
Amino Acids and Disease: The Dark Side
Imbalances in amino acid levels or deficiencies in specific amino acids can contribute to various health problems. Examples include:
-
Phenylketonuria (PKU): A genetic disorder resulting in the inability to metabolize phenylalanine, leading to its accumulation in the blood and causing neurological damage.
-
Maple syrup urine disease (MSUD): A genetic disorder involving the inability to metabolize branched-chain amino acids (leucine, isoleucine, and valine), resulting in the accumulation of these amino acids in the blood and urine, causing neurological damage.
-
Alkaptonuria: A rare genetic disorder characterized by the body's inability to break down tyrosine and phenylalanine. This leads to an accumulation of homogentisic acid, which causes dark pigmentation of the urine and can cause serious damage to the joints and heart.
-
Certain cancers: Dysregulation of amino acid metabolism has been linked to the development and progression of various cancers.
Conclusion: The Profound Importance of Amino Acids
Amino acids, these simple yet remarkably versatile molecules, are integral to life. Their roles extend far beyond their contribution to protein synthesis, impacting numerous metabolic processes and having profound implications for human health. Understanding the structure, properties, and functions of amino acids is crucial for appreciating the complexity of biological systems and for advancing our understanding of human health and disease. Further research continues to unravel the intricate roles of amino acids in maintaining health and combating disease, highlighting the enduring significance of these fundamental building blocks of life.
Latest Posts
Related Post
Thank you for visiting our website which covers about Amino Acids Composed Of Carbon Hydrogen Nitrogen And Oxygen . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.