All Of The Following Nucleotide Bases Are Pyrimidines Except
News Leon
Mar 22, 2025 · 7 min read
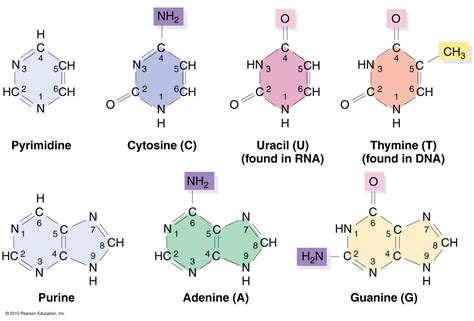
Table of Contents
All of the following nucleotide bases are pyrimidines except… Adenine!
Understanding the fundamental building blocks of DNA and RNA is crucial for comprehending the intricacies of life itself. These molecules, the blueprints of life, are constructed from nucleotides, each composed of a sugar, a phosphate group, and a nitrogenous base. These nitrogenous bases are categorized into two groups: purines and pyrimidines. This article will delve deep into the world of nucleotide bases, focusing on the key difference between purines and pyrimidines, clarifying the statement "All of the following nucleotide bases are pyrimidines except...", and exploring their vital roles in molecular biology.
Purines vs. Pyrimidines: A Structural Distinction
The core difference between purines and pyrimidines lies in their chemical structure. This structural difference dictates their function and how they interact within the DNA double helix and RNA single helix.
Pyrimidines: These bases are characterized by a single six-membered heterocyclic aromatic ring containing two nitrogen atoms. Think of it as a single hexagonal structure with two nitrogen atoms embedded within the carbon ring. The pyrimidine bases found in DNA and RNA are:
- Cytosine (C): Present in both DNA and RNA, cytosine pairs with guanine (G) through three hydrogen bonds. It plays a crucial role in gene expression and regulation.
- Thymine (T): Found exclusively in DNA, thymine pairs with adenine (A) through two hydrogen bonds. Its presence is a defining characteristic of DNA.
- Uracil (U): Found exclusively in RNA, uracil replaces thymine and pairs with adenine (A) through two hydrogen bonds. Its presence distinguishes RNA from DNA.
Purines: In contrast to pyrimidines, purines possess a more complex structure. They are characterized by a fused ring system composed of a six-membered ring attached to a five-membered ring, both containing nitrogen atoms. This creates a larger, more complex structure compared to pyrimidines. The purine bases are:
- Adenine (A): Present in both DNA and RNA, adenine pairs with thymine (T) in DNA and uracil (U) in RNA. Its pairing is crucial for maintaining the double helix structure in DNA and the overall structure of RNA.
- Guanine (G): Present in both DNA and RNA, guanine pairs with cytosine (C) through three hydrogen bonds. Its strong pairing contributes to the stability of DNA and RNA structures.
Therefore, the statement "All of the following nucleotide bases are pyrimidines except..." is completed by Adenine. Adenine is a purine, not a pyrimidine, distinguishing it from cytosine, thymine, and uracil.
The Significance of Base Pairing
The specific pairing of purines with pyrimidines (A with T/U and G with C) is fundamental to the double helix structure of DNA and the various structures adopted by RNA. This complementary base pairing is crucial for several key processes:
-
DNA Replication: During replication, the DNA double helix unwinds, and each strand serves as a template for the synthesis of a new complementary strand. The precise pairing of bases ensures accurate duplication of genetic information.
-
Transcription: The process of transcription involves the synthesis of RNA from a DNA template. Again, complementary base pairing guides the accurate copying of genetic information from DNA to RNA.
-
Translation: In translation, the sequence of codons in mRNA determines the sequence of amino acids in a protein. The interaction between mRNA codons and tRNA anticodons relies on complementary base pairing.
The consistent and precise pairing of purines and pyrimidines ensures the fidelity of genetic information transfer and is essential for the proper functioning of cellular processes. Any error in base pairing can lead to mutations, potentially causing genetic disorders or diseases.
The Chemical Properties of Pyrimidine Bases
The chemical properties of pyrimidines directly influence their behavior within the DNA and RNA structures. Several aspects are noteworthy:
-
Hydrogen Bonding: The presence of oxygen and nitrogen atoms in the pyrimidine ring allows for the formation of hydrogen bonds with purines. These hydrogen bonds are relatively weak but collectively contribute significantly to the stability of the DNA double helix and RNA structures. The number of hydrogen bonds (two for A-T/U and three for G-C) influences the strength of the base pairs.
-
Keto-Enol Tautomerism: Pyrimidine bases can exist in different tautomeric forms, depending on the pH of the environment. Keto-enol tautomerism can affect base pairing and potentially lead to mutations if the wrong tautomer is involved in replication or transcription.
-
UV Absorption: Pyrimidine bases strongly absorb ultraviolet (UV) light. This property is utilized in various techniques used in molecular biology, such as spectrophotometry, to quantify DNA and RNA. Exposure to excessive UV radiation can lead to the formation of pyrimidine dimers, causing DNA damage and potentially leading to mutations.
Understanding the chemical properties of pyrimidine bases is critical for comprehending their role in various biological processes and their susceptibility to damage from external factors.
The Roles of Pyrimidine Bases in Biological Processes
Pyrimidine bases are not merely structural components of nucleic acids; they play active roles in diverse cellular processes. Their roles extend beyond simply forming the building blocks of DNA and RNA.
-
Gene Regulation: Cytosine can be methylated, a process that affects gene expression. Methylation of cytosine plays a crucial role in regulating gene activity and is involved in various cellular processes, including development and disease.
-
Signal Transduction: Modified pyrimidine bases can serve as signaling molecules involved in cell communication and regulation. These modifications often affect the interaction of proteins with nucleic acids and influence biological processes.
-
Enzyme Activity: Some enzymes require specific pyrimidine bases or their derivatives as cofactors, thereby highlighting their involvement in catalysis and metabolism.
These functions demonstrate that pyrimidine bases are not simply passive structural components but actively participate in regulating various biological functions.
Clinical Significance of Pyrimidine Metabolism Disorders
Disruptions in pyrimidine metabolism can lead to a range of inherited disorders. These disorders often result from deficiencies in enzymes involved in the synthesis or degradation of pyrimidine bases. These disorders can manifest in various ways, affecting different systems of the body. Examples include:
-
Orotic aciduria: This disorder is characterized by an accumulation of orotic acid in the body, a byproduct of pyrimidine metabolism. It often leads to developmental delays and anemia.
-
Hereditary orotic aciduria: This rarer condition involves genetic defects affecting enzymes in the pyrimidine synthesis pathway, leading to severe anemia, developmental issues, and mental retardation.
These conditions underscore the critical importance of proper pyrimidine metabolism for overall health and development.
Pyrimidines in Research and Therapeutics
Pyrimidine bases and their analogs have found significant applications in research and therapeutics. Their uses range from research tools to treatments for various diseases.
-
Research Tools: Labeled pyrimidine bases are used as tracers to study DNA and RNA metabolism. Understanding these processes allows researchers to gain insights into the cell's workings and disease mechanisms.
-
Anticancer Drugs: Several anticancer drugs are analogs of pyrimidine bases. These analogs interfere with DNA replication and repair, ultimately inhibiting the growth of cancer cells. 5-fluorouracil (5-FU) is a classic example used in cancer chemotherapy.
-
Antiviral Drugs: Some antiviral drugs target viral DNA or RNA polymerases, exploiting their reliance on pyrimidine bases for replication. These drugs work by interfering with the viral replication cycle, inhibiting viral growth.
The ongoing research on pyrimidines and their derivatives continues to expand our understanding of their biological functions and their therapeutic potential in various diseases.
Conclusion
In summary, the statement "All of the following nucleotide bases are pyrimidines except..." is correctly completed with adenine. Adenine, a purine base, contrasts structurally and functionally with the pyrimidine bases—cytosine, thymine, and uracil. The distinction between purines and pyrimidines is critical to understanding DNA and RNA structure, function, and replication. The unique chemical properties and biological roles of pyrimidine bases highlight their significance in various cellular processes and their implications in human health and disease. Further research into pyrimidine metabolism and its interactions will continue to provide valuable insights into the intricate world of molecular biology and contribute to the development of new therapeutic strategies.
Latest Posts
Latest Posts
-
Which Of The Following Are Rational Numbers
Mar 22, 2025
-
What Was One Of The Goals Of The Muslim League
Mar 22, 2025
-
How Many Km Is 1 7 Miles
Mar 22, 2025
-
Ode To The West Wind Meaning
Mar 22, 2025
-
How Many Kilograms In 5000 Grams
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about All Of The Following Nucleotide Bases Are Pyrimidines Except . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
