Acetic Acid And Ethyl Alcohol Reaction
News Leon
Mar 25, 2025 · 6 min read
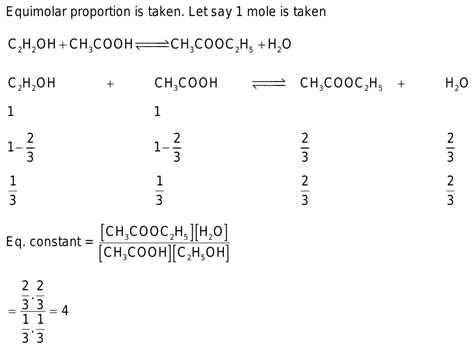
Table of Contents
Acetic Acid and Ethyl Alcohol Reaction: Esterification and its Applications
The reaction between acetic acid (CH₃COOH) and ethyl alcohol (CH₃CH₂OH) is a classic example of esterification, a crucial process in organic chemistry with widespread applications in various industries. This reaction produces ethyl acetate, a sweet-smelling ester commonly used as a solvent and in the production of perfumes, nail polish remover, and other products. Understanding the mechanism, kinetics, and applications of this reaction is essential for students and professionals in chemistry and related fields.
Understanding the Esterification Reaction
Esterification is a reversible reaction where a carboxylic acid reacts with an alcohol in the presence of an acid catalyst to form an ester and water. In the specific case of acetic acid and ethyl alcohol, the reaction can be represented as follows:
CH₃COOH + CH₃CH₂OH ⇌ CH₃COOCH₂CH₃ + H₂O
Acetic acid (also known as ethanoic acid), a weak organic acid, provides the carboxyl group (-COOH). Ethyl alcohol (also known as ethanol), a primary alcohol, provides the hydroxyl group (-OH). The acid catalyst, typically sulfuric acid (H₂SO₄), helps protonate the carbonyl oxygen of the carboxylic acid, making it more susceptible to nucleophilic attack by the alcohol.
The Mechanism of Esterification
The esterification reaction proceeds through several steps:
-
Protonation of the carbonyl oxygen: The acid catalyst protonates the carbonyl oxygen of the acetic acid, making it a better electrophile. This increases the positive charge on the carbonyl carbon, making it more attractive to the nucleophile.
-
Nucleophilic attack by the alcohol: The oxygen atom of the ethyl alcohol, acting as a nucleophile, attacks the carbonyl carbon of the protonated acetic acid. This forms a tetrahedral intermediate.
-
Proton transfer: A proton is transferred from the hydroxyl group of the tetrahedral intermediate to one of the oxygen atoms.
-
Elimination of water: A molecule of water is eliminated, regenerating the carbonyl group and forming the ester linkage.
-
Deprotonation: The protonated ester is deprotonated by a water molecule or a base, yielding the final ester product, ethyl acetate.
This mechanism highlights the crucial role of the acid catalyst in facilitating the reaction. Without the catalyst, the reaction would be significantly slower.
Factors Affecting the Esterification Reaction
Several factors can influence the rate and yield of the esterification reaction:
-
Temperature: Higher temperatures generally increase the reaction rate, although excessively high temperatures can lead to side reactions or decomposition of reactants.
-
Concentration of reactants: Increasing the concentration of either acetic acid or ethyl alcohol will increase the reaction rate, according to the principles of mass action.
-
Acid catalyst concentration: The concentration of the acid catalyst also affects the reaction rate. However, an excessively high concentration of the catalyst can lead to unwanted side reactions.
-
Water content: Since esterification is a reversible reaction, the presence of water can shift the equilibrium towards the reactants. Removing water from the reaction mixture, for example, by using a Dean-Stark apparatus, can drive the reaction towards the formation of the ester and improve the yield.
-
Nature of the alcohol: Primary alcohols generally react faster than secondary alcohols, which react faster than tertiary alcohols. The steric hindrance around the hydroxyl group plays a significant role in determining the reaction rate.
Kinetics of the Esterification Reaction
The kinetics of esterification is complex and depends on several factors, including the concentrations of the reactants and the catalyst. The reaction is generally considered to be second-order, with the rate being proportional to the concentrations of both the acetic acid and the ethyl alcohol. However, the exact rate law can vary depending on the specific reaction conditions and the presence of excess reactants. Detailed kinetic studies often involve measuring the concentration of the reactants or products over time using techniques like titration or chromatography. These studies can help in optimizing the reaction conditions to maximize the yield of the ester.
Applications of Ethyl Acetate
Ethyl acetate, the product of the acetic acid and ethyl alcohol reaction, is a versatile compound with numerous applications in various industries:
-
Solvent: Ethyl acetate is a widely used solvent due to its excellent solubility properties, low toxicity, and relatively low cost. It's used as a solvent in the production of paints, coatings, adhesives, and inks.
-
Perfume and Flavor Industry: Ethyl acetate contributes a pleasant fruity aroma and is used in the production of perfumes, cosmetics, and food flavorings. Its sweet, slightly fruity odor makes it a popular ingredient in many artificial fruit flavors.
-
Nail Polish Remover: Many commercially available nail polish removers use ethyl acetate as their primary solvent because of its ability to dissolve the common polymers used in nail polish.
-
Extraction: Ethyl acetate is a common solvent in extraction processes, particularly in the pharmaceutical and chemical industries, due to its ability to selectively extract certain compounds from complex mixtures.
-
Cleaning Agent: Ethyl acetate's solvent properties make it suitable for cleaning applications, particularly for removing grease and oil.
Industrial Production of Ethyl Acetate
The industrial production of ethyl acetate typically involves a continuous process using a large reactor. The reaction is carried out in the presence of an acid catalyst, such as sulfuric acid or p-toluenesulfonic acid, and water is removed from the reaction mixture to shift the equilibrium towards the product. The process often involves distillation to separate the ethyl acetate from the reaction mixture and recycle unreacted starting materials. Various optimization strategies are employed to maximize the yield and efficiency of the process.
Safety Precautions
When handling acetic acid, ethyl alcohol, and ethyl acetate, it is crucial to follow appropriate safety precautions. Acetic acid is a corrosive substance and can cause skin irritation and burns. Ethyl alcohol is flammable and should be kept away from ignition sources. Ethyl acetate is also flammable and has a relatively low flash point. Always wear appropriate personal protective equipment, such as gloves, safety goggles, and a lab coat, when working with these chemicals. Work in a well-ventilated area to avoid inhaling fumes. Proper disposal procedures should be followed to ensure environmental safety.
Conclusion
The reaction between acetic acid and ethyl alcohol to produce ethyl acetate is a fundamental reaction in organic chemistry with significant industrial applications. Understanding the mechanism, kinetics, and factors affecting the reaction is essential for optimizing the process and producing high yields of ethyl acetate. This versatile ester plays a crucial role in various industries, from solvents and coatings to perfumes and food flavorings. Safety precautions are paramount when handling the reactants and products involved in this reaction. Future research may focus on developing more sustainable and efficient methods for ethyl acetate production, potentially employing greener catalysts and solvents. Further investigation into the reaction kinetics under different conditions can also contribute to optimizing the process for various applications. The continuous refinement of this classic reaction ensures its continued relevance in chemical synthesis and industrial production.
Latest Posts
Latest Posts
-
Organelle That Packages And Delivers Proteins
Mar 28, 2025
-
A Dilute Ferrous Sulphate Solution Was Gradually
Mar 28, 2025
-
Most Chemical Digestion Takes Place In The
Mar 28, 2025
-
What Is The Conjugate Base Of Hso4
Mar 28, 2025
-
What Is The Function Of Areolar Tissue
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Acetic Acid And Ethyl Alcohol Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
