According To The Bronsted-lowry Definition A Base Is
News Leon
Apr 06, 2025 · 6 min read
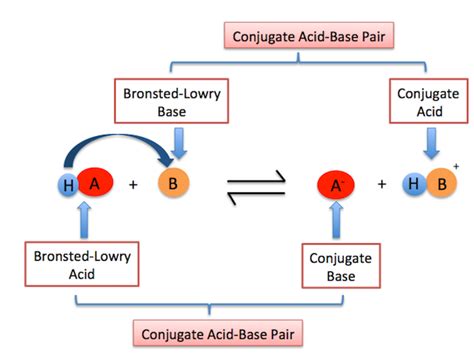
Table of Contents
According to the Brønsted-Lowry Definition, a Base Is...
The Brønsted-Lowry definition of acids and bases significantly expands our understanding beyond the limitations of the Arrhenius definition. While Arrhenius focused solely on the production of hydrogen (H⁺) and hydroxide (OH⁻) ions in aqueous solutions, Brønsted and Lowry broadened the scope to encompass proton transfer reactions, regardless of the solvent. This article delves into the Brønsted-Lowry definition of a base, exploring its nuances, applications, and comparing it to other acid-base theories.
Understanding the Brønsted-Lowry Definition
According to the Brønsted-Lowry definition, a base is a substance that accepts a proton (H⁺) from another substance. This seemingly simple definition unlocks a much wider range of chemical reactions considered acid-base interactions. Crucially, this definition doesn't require the presence of water; proton transfer can occur in various solvents or even in the gas phase.
The key to understanding this definition lies in the concept of proton transfer. An acid, in the Brønsted-Lowry framework, is a proton donor. When an acid interacts with a base, it donates a proton to the base, which accepts it. This exchange forms the core of a Brønsted-Lowry acid-base reaction.
Key Differences from the Arrhenius Definition
The Arrhenius definition, while foundational, is limited. It defines a base as a substance that produces hydroxide ions (OH⁻) in water. This restricts the definition to aqueous solutions and excludes many compounds that exhibit basic behavior in non-aqueous solvents or through different mechanisms. The Brønsted-Lowry definition overcomes this limitation by focusing on the proton transfer mechanism, irrespective of the solvent.
For example, ammonia (NH₃) acts as a base by accepting a proton from water:
NH₃(aq) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq)
In this reaction, ammonia accepts a proton from water, becoming the ammonium ion (NH₄⁺). Water, in turn, acts as the acid, donating a proton and becoming a hydroxide ion (OH⁻). Note that, while hydroxide ions are formed, the reaction's essence lies in the proton transfer, aligning perfectly with the Brønsted-Lowry definition.
Types of Brønsted-Lowry Bases
Brønsted-Lowry bases encompass a diverse range of chemical species, each with its own mechanism for accepting protons. Let's explore some common types:
1. Hydroxide Ions (OH⁻):
These are the classic Arrhenius bases and remain a crucial subset within the Brønsted-Lowry framework. They readily accept protons to form water:
OH⁻(aq) + H⁺(aq) → H₂O(l)
2. Ammonia and Amines:
Ammonia (NH₃) and amines (organic compounds derived from ammonia by replacing one or more hydrogen atoms with alkyl or aryl groups) are excellent examples of Brønsted-Lowry bases. Their lone pair of electrons on the nitrogen atom readily accepts a proton:
CH₃NH₂(aq) + H₂O(l) ⇌ CH₃NH₃⁺(aq) + OH⁻(aq) (Methylamine acting as a base)
3. Carboxylic Acid Anions:
The conjugate bases of carboxylic acids (e.g., acetate ion, CH₃COO⁻) are Brønsted-Lowry bases. They can accept a proton to reform the carboxylic acid.
CH₃COO⁻(aq) + H⁺(aq) → CH₃COOH(aq)
4. Carbonate and Bicarbonate Ions:
These ions are crucial in buffering systems and readily accept protons.
CO₃²⁻(aq) + H⁺(aq) → HCO₃⁻(aq) HCO₃⁻(aq) + H⁺(aq) → H₂CO₃(aq)
5. Water as a Base:
Water itself can act as both a Brønsted-Lowry acid and a base, a property known as amphiprotism or amphoterism. This is evident in the self-ionization of water:
2H₂O(l) ⇌ H₃O⁺(aq) + OH⁻(aq)
In this reaction, one water molecule acts as an acid (donating a proton), and another acts as a base (accepting a proton).
Conjugate Acid-Base Pairs
A crucial concept within the Brønsted-Lowry theory is the formation of conjugate acid-base pairs. When a base accepts a proton, it forms its conjugate acid. Conversely, when an acid donates a proton, it forms its conjugate base.
Consider the reaction between ammonia and water again:
NH₃(aq) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq)
- NH₃ is the base, and NH₄⁺ is its conjugate acid.
- H₂O is the acid, and OH⁻ is its conjugate base.
Notice that a conjugate acid-base pair differs by only a single proton (H⁺). This relationship is fundamental to understanding acid-base equilibrium and calculations.
Strength of Brønsted-Lowry Bases
The strength of a Brønsted-Lowry base is determined by its ability to accept a proton. Strong bases readily accept protons, while weak bases accept protons less readily. This is often quantified using the base dissociation constant (Kb), which is analogous to the acid dissociation constant (Ka). A higher Kb value indicates a stronger base.
The strength of a base is also inversely related to the strength of its conjugate acid. A strong base has a weak conjugate acid, and vice versa. This relationship is crucial for predicting the direction of acid-base reactions.
Applications of the Brønsted-Lowry Definition
The Brønsted-Lowry definition has far-reaching applications in various fields:
- Chemistry: Understanding acid-base reactions in non-aqueous solvents, predicting reaction outcomes, and calculating equilibrium constants.
- Biochemistry: Explaining the behavior of amino acids, proteins, and nucleic acids, which often act as Brønsted-Lowry acids and bases.
- Environmental Science: Analyzing acid rain, water treatment processes, and soil chemistry, where proton transfer reactions are vital.
- Medicine: Understanding the role of pH in biological systems and the design of buffers for drug delivery and therapy.
Comparison with Other Acid-Base Theories
While the Brønsted-Lowry theory is widely used, it's important to note other acid-base theories:
- Arrhenius Theory: As discussed, this is a more limited theory restricted to aqueous solutions and hydroxide ion production.
- Lewis Theory: This theory defines a base as an electron pair donor. It's broader than Brønsted-Lowry, encompassing reactions that don't involve proton transfer but involve the sharing of electron pairs. While Brønsted-Lowry bases are also Lewis bases, the reverse is not always true.
Conclusion
The Brønsted-Lowry definition of a base provides a comprehensive and versatile framework for understanding a vast array of chemical reactions involving proton transfer. Its emphasis on the proton-accepting ability of a substance, regardless of the solvent, allows for a more nuanced and accurate description of acid-base chemistry than the earlier Arrhenius theory. The concepts of conjugate acid-base pairs and the relative strengths of bases are crucial for applying this definition effectively, impacting various scientific disciplines, from biochemistry to environmental science. While other theories exist, the Brønsted-Lowry model remains a cornerstone of modern acid-base chemistry. Understanding its nuances is essential for anyone seeking a deep understanding of chemical reactions and their applications.
Latest Posts
Latest Posts
-
What Are The Units Of Potential Energy
Apr 09, 2025
-
Is Dry Ice A Compound Element Or Mixture
Apr 09, 2025
-
Vapour Pressure Of Water In Torr
Apr 09, 2025
-
Which Of The Following Is Not True Regarding Viruses
Apr 09, 2025
-
What Is One Difference Between A Mixture And A Compound
Apr 09, 2025
Related Post
Thank you for visiting our website which covers about According To The Bronsted-lowry Definition A Base Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.