A Solution Contains Dissolved Substances Called
News Leon
Mar 20, 2025 · 6 min read
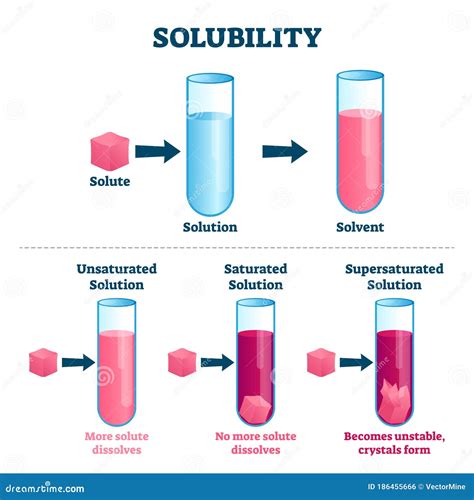
Table of Contents
A Solution Contains Dissolved Substances Called: Solutes and Their Impact
A solution, in the simplest terms, is a homogeneous mixture composed of two or more substances. This means the mixture is uniform throughout; you won't find pockets of one substance concentrated in one area and another in a different area. The key to understanding a solution lies in recognizing its constituent parts: the solvent and the solute. This article delves deep into the nature of solutes, their properties, and their profound impact on the characteristics of the resulting solution.
Understanding Solutes: The Dissolved Substances
The substances that dissolve in a solution are called solutes. They can be solids, liquids, or gases, and their dissolution in the solvent is the defining characteristic of a solution. Think of making sweet tea: the sugar (solute) dissolves in the water (solvent) to form a homogeneous mixture. The quantity of solute present relative to the solvent significantly impacts the properties of the solution.
Types of Solutes and their Properties
The nature of the solute plays a crucial role in how it interacts with the solvent and the overall properties of the solution. Some key characteristics influencing solute behavior include:
-
Polarity: Polar solutes, possessing a partial positive and negative charge, tend to dissolve well in polar solvents (like water), while nonpolar solutes dissolve better in nonpolar solvents (like oil). This principle is often summarized as "like dissolves like."
-
Solubility: This refers to the maximum amount of solute that can dissolve in a given amount of solvent at a specific temperature and pressure. Solubility varies significantly depending on the solute and solvent. Some substances are highly soluble (e.g., table salt in water), while others are sparingly soluble or virtually insoluble.
-
Molecular Weight: The size and mass of the solute molecules influence how easily they can be dispersed within the solvent. Larger molecules generally have lower solubility compared to smaller molecules.
-
Temperature: The solubility of many solutes increases with temperature. This is because higher temperatures provide more kinetic energy to the solute particles, enabling them to overcome intermolecular forces and dissolve more effectively.
-
Pressure: Pressure primarily affects the solubility of gaseous solutes. Increased pressure generally increases the solubility of gases in liquids. This is why carbonated beverages are kept under pressure—to maintain the dissolved carbon dioxide.
Examples of Common Solutes
We encounter numerous examples of solutes in our daily lives, often without even realizing it. Consider these everyday instances:
-
Saltwater: Sodium chloride (table salt) acts as the solute dissolved in the water (solvent).
-
Sweet Tea/Coffee: Sugar or sweeteners dissolve as solutes in the water-based beverage.
-
Air: Various gases, including oxygen, nitrogen, and carbon dioxide, act as solutes dissolved in the primary component, nitrogen.
-
Alloys: In metallurgy, one metal acts as the solute, dissolving in another metal which serves as the solvent. Brass, for example, is an alloy of copper (solvent) and zinc (solute).
-
Medicines: Many medications are formulated as solutions, with the active pharmaceutical ingredient (API) acting as the solute dissolved in a suitable solvent.
The Role of Solutes in Solution Properties
The presence of solutes profoundly alters the properties of the resulting solution compared to the pure solvent. Several key properties are affected:
-
Boiling Point Elevation: Adding a non-volatile solute to a solvent increases its boiling point. This phenomenon, known as boiling point elevation, is directly proportional to the concentration of the solute. The more solute, the higher the boiling point.
-
Freezing Point Depression: Conversely, adding a solute lowers the freezing point of the solvent. This is known as freezing point depression and is also directly proportional to the solute concentration. This is why saltwater has a lower freezing point than freshwater.
-
Osmotic Pressure: Osmotic pressure refers to the pressure required to prevent the flow of solvent across a semipermeable membrane from a region of low solute concentration to a region of high solute concentration. Solutes contribute significantly to the osmotic pressure of a solution.
-
Vapor Pressure Lowering: The presence of a non-volatile solute reduces the vapor pressure of the solvent. This is because the solute molecules occupy space at the surface of the liquid, reducing the number of solvent molecules that can escape into the gaseous phase.
Concentration and its Significance
The concentration of a solute within a solution is crucial in determining its properties. Concentration is typically expressed as the amount of solute present per unit volume or mass of the solution. Several ways to express concentration include:
-
Molarity (M): Moles of solute per liter of solution.
-
Molality (m): Moles of solute per kilogram of solvent.
-
Percent by Mass (% w/w): Grams of solute per 100 grams of solution.
-
Percent by Volume (% v/v): Milliliters of solute per 100 milliliters of solution.
-
Parts per Million (ppm) and Parts per Billion (ppb): Used for extremely low concentrations.
Understanding the concentration of a solute is critical for many applications, from preparing chemical reagents in laboratories to formulating medications and monitoring pollutants in the environment.
Applications and Importance of Solutes
The concept of solutes and solutions is fundamental to numerous scientific disciplines and practical applications. Here are a few examples:
-
Medicine: Drug delivery often relies on solutions, where the active pharmaceutical ingredient is the solute dissolved in a suitable solvent. Understanding solute solubility and concentration is crucial for ensuring appropriate drug dosage and efficacy.
-
Chemistry: Solutions are essential in chemical reactions, with solutes providing the reactants. The concentration of solutes often dictates the reaction rate and yield.
-
Biology: Biological systems heavily rely on solutions. Cells are surrounded by fluids that contain dissolved solutes like ions, sugars, and proteins. The movement of solutes across cell membranes is critical for various biological processes.
-
Environmental Science: Understanding solute concentrations in water bodies is essential for monitoring water quality and assessing environmental pollution. Solutes like heavy metals and pesticides can have harmful effects on ecosystems and human health.
-
Food Science: Many food products are solutions or suspensions. The solutes contribute to taste, texture, and preservation. The careful control of solute concentration is essential for maintaining food quality and safety.
Beyond Simple Solutions: Complex Systems
While we've focused on simple solutions, many systems in nature and industry are far more complex. These can include:
-
Colloids: These are mixtures where the solute particles are larger than those in a true solution but not large enough to settle out under gravity. Examples include milk and fog.
-
Suspensions: These are mixtures where the solute particles are larger and will eventually settle out. Muddy water is an example of a suspension.
Understanding the distinction between solutions, colloids, and suspensions is important for various applications, particularly in material science and environmental engineering.
Conclusion: The Ubiquity of Solutes
Solutes are the essential components that define solutions, profoundly impacting their properties and playing a crucial role in a vast array of natural and technological processes. From the simplest saltwater solution to the complex biochemical reactions within living organisms, the presence and behavior of solutes are fundamental to our understanding of the world around us. Continued research into solute behavior and interactions will continue to yield advancements in numerous fields, emphasizing the ongoing importance of this seemingly simple concept. The interplay between solutes and solvents is a testament to the complex yet beautiful organization of matter. Understanding this relationship is key to unlocking deeper insights into various scientific and technological advancements.
Latest Posts
Latest Posts
-
There Are Two Forces On The 2 00 Kg Box
Mar 21, 2025
-
The Electric Potential V In The Space Between
Mar 21, 2025
-
A Particle Of Charge Q Is Fixed At Point P
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about A Solution Contains Dissolved Substances Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
