A Metal Crystallizes In A Face-centered Cubic Structure
News Leon
Mar 24, 2025 · 7 min read
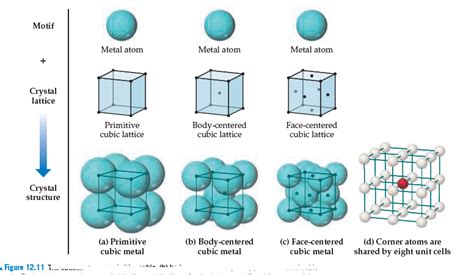
Table of Contents
A Metal Crystallizes in a Face-Centered Cubic Structure: A Deep Dive into FCC Crystals
The world of materials science is replete with fascinating structures and properties, and among the most prevalent and significant is the face-centered cubic (FCC) crystal structure. Many metals, crucial to various industries, adopt this arrangement of atoms. This article delves into the intricacies of the FCC structure, exploring its geometry, properties, and significance in material science and engineering. We will examine how the arrangement of atoms in this structure dictates the macroscopic properties of the material, making it suitable for a wide range of applications.
Understanding the Face-Centered Cubic (FCC) Structure
The face-centered cubic structure, as its name suggests, is a type of cubic crystal system. Imagine a cube; in an FCC structure, atoms are located at each of the eight corners of the cube and at the center of each of the six faces. This arrangement leads to a highly efficient packing of atoms, resulting in a coordination number of 12 – each atom is surrounded by 12 nearest neighbors. This high coordination number contributes significantly to the properties of FCC metals.
Atom Positions and Unit Cell
The unit cell, the smallest repeating unit of the crystal structure, is crucial for understanding the FCC lattice. The unit cell for an FCC structure is a cube, with atoms at each corner and the center of each face. It's important to note that the atoms at the corners are shared by eight adjacent unit cells, while those on the faces are shared by two. This sharing needs to be considered when calculating the number of atoms per unit cell.
Calculating the number of atoms per unit cell:
- Corner atoms: 8 corner atoms × (1/8 atom/corner) = 1 atom
- Face-centered atoms: 6 face-centered atoms × (1/2 atom/face) = 3 atoms
- Total atoms per unit cell: 1 + 3 = 4 atoms
This calculation reveals that there are four atoms per unit cell in an FCC structure. This seemingly simple fact has profound implications for the material's density and other properties.
Atomic Packing Factor (APF)
The atomic packing factor (APF) is a crucial parameter that quantifies the efficiency of atomic packing within a crystal structure. It represents the fraction of the unit cell volume occupied by atoms, assuming the atoms are hard spheres. For FCC structures, the APF is remarkably high, at approximately 0.74. This high APF signifies a highly efficient packing arrangement, contributing to the relatively high density observed in many FCC metals.
Calculating the Atomic Packing Factor:
The calculation of APF involves determining the volume occupied by the atoms within the unit cell and dividing it by the total volume of the unit cell. The derivation is complex and requires geometrical considerations, but the result consistently points to an APF of 0.74 for FCC structures. This high packing efficiency is a key characteristic distinguishing FCC from other crystal structures like body-centered cubic (BCC) or hexagonal close-packed (HCP).
Properties of FCC Metals
The unique atomic arrangement in the FCC structure directly influences its macroscopic properties. These properties make FCC metals highly desirable for various applications. Let's explore some of these key properties:
High Ductility and Malleability
FCC metals are renowned for their high ductility and malleability. Ductility refers to a material's ability to deform under tensile stress, while malleability refers to its ability to deform under compressive stress. The ability of atoms in the FCC structure to easily slip past each other along close-packed planes facilitates these plastic deformation mechanisms. This makes FCC metals easily workable and shapeable, vital characteristics in manufacturing processes.
High Density
As mentioned earlier, the high atomic packing factor (APF) of 0.74 results in a high density for FCC metals compared to other crystal structures. This high density is beneficial in applications requiring high mass or weight concentration within a given volume.
Relatively High Melting Point
While the melting point varies across different FCC metals, generally, they exhibit relatively high melting points compared to some other crystal structures. This characteristic is attributed to the strong metallic bonding and high coordination number in the FCC lattice, requiring more energy to overcome the attractive forces between atoms and transition to the liquid state.
Slip Systems and Deformation Mechanisms
The FCC crystal structure possesses multiple slip systems, which are planes and directions along which dislocations can easily move. Dislocations are crystal imperfections that play a key role in plastic deformation. The abundance of slip systems in FCC structures allows for relatively easy plastic deformation under applied stress, contributing to the observed ductility and malleability. This characteristic is crucial for applications involving forming, drawing, and other shaping processes.
Isotropy
Many FCC metals exhibit a degree of isotropy, meaning their properties are relatively similar in all directions. This is a consequence of the symmetrical arrangement of atoms in the FCC lattice. However, it's important to note that perfect isotropy is rare, and some anisotropic properties might still exist depending on the specific material and its processing.
Examples of FCC Metals
Numerous metals crystallize in the FCC structure, including:
- Aluminum (Al): Widely used in transportation, packaging, and construction due to its lightweight and corrosion-resistant nature.
- Copper (Cu): Excellent electrical and thermal conductivity makes it essential in electrical wiring and heat exchangers.
- Gold (Au): Highly prized for its inertness, ductility, and malleability, used in jewelry and electronics.
- Silver (Ag): Similar to copper, it possesses high electrical and thermal conductivity and is used in various electronic applications.
- Nickel (Ni): Known for its strength, corrosion resistance, and magnetic properties, finds applications in various alloys and catalysts.
- Lead (Pb): Traditionally used in batteries and radiation shielding due to its high density and relative inertness.
- Platinum (Pt): Valuable for its catalytic properties and corrosion resistance, used in automotive catalysts and jewelry.
- Palladium (Pd): Also a valuable catalyst, used in catalytic converters and chemical processes.
Applications of FCC Metals
The unique combination of properties possessed by FCC metals makes them invaluable across various industries. Their applications span a wide range of fields:
Automotive Industry
FCC metals, especially aluminum and steel alloys, are essential components in modern vehicles. Aluminum's lightweight nature contributes to improved fuel efficiency, while steel alloys, often containing FCC components, offer strength and durability. Their malleability allows for complex shaping in automotive body panels and other components.
Aerospace Industry
Similar to the automotive industry, the aerospace industry leverages the lightweight nature and high strength of FCC metals (like aluminum alloys and titanium alloys, although titanium is HCP) to create lighter and more fuel-efficient aircraft and spacecraft. Their corrosion resistance is also crucial for enduring harsh environmental conditions.
Electronics Industry
Copper and silver, both FCC metals, are indispensable in the electronics industry due to their exceptional electrical conductivity. They are used in wiring, printed circuit boards, and various electronic components. Gold's inertness and conductivity also contribute to its use in electronic contacts.
Packaging Industry
Aluminum's corrosion resistance, formability, and recyclability make it ideal for various packaging applications. Aluminum cans, foil, and other packaging materials are ubiquitous examples of FCC metal usage.
Medical Industry
Certain biocompatible FCC metals and alloys are used in medical implants and devices due to their inertness and ability to integrate with the body. Their biocompatibility is crucial for long-term implant stability and to prevent adverse reactions.
Beyond the Basics: Alloying and Strengthening Mechanisms
The properties of FCC metals can be further tailored by alloying – the process of adding other elements to modify their characteristics. Alloying can significantly enhance strength, hardness, corrosion resistance, and other properties. Several strengthening mechanisms are employed in FCC alloys:
- Solid Solution Strengthening: Adding alloying elements that dissolve in the FCC lattice can disrupt the regular atomic arrangement, hindering dislocation movement and increasing strength.
- Precipitation Hardening: Introducing precipitates (small particles of a second phase) within the FCC matrix can impede dislocation motion, significantly increasing strength and hardness.
- Strain Hardening (Work Hardening): Deforming the metal plastically introduces dislocations, which interact and hinder each other's motion, leading to increased strength. However, this also reduces ductility.
Conclusion
The face-centered cubic crystal structure is a fundamental aspect of materials science, governing the properties of many technologically important metals. The high atomic packing factor, multiple slip systems, and the possibility of alloying to tailor properties make FCC metals extremely versatile and applicable across diverse sectors. Understanding the intricacies of the FCC structure and its influence on material behavior is crucial for designing and manufacturing a vast array of products, from vehicles and aircraft to electronic devices and medical implants. Further research into manipulating and improving the properties of FCC metals continues to drive innovation and technological advancements across various fields. The exploration and understanding of this structure are continually evolving, promising further discoveries and applications in the future.
Latest Posts
Latest Posts
-
4 Functions Of A Political Party
Mar 26, 2025
-
Where Does Mitosis Take Place In The Body
Mar 26, 2025
-
A Proton Travels Through Uniform Magnetic And Electric Fields
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about A Metal Crystallizes In A Face-centered Cubic Structure . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
