A Compound Contains Only Carbon Hydrogen And Oxygen
News Leon
Mar 23, 2025 · 7 min read
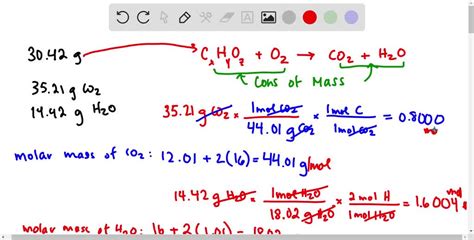
Table of Contents
- A Compound Contains Only Carbon Hydrogen And Oxygen
- Table of Contents
- A Compound Contains Only Carbon, Hydrogen, and Oxygen: Exploring the World of Organic Chemistry
- Understanding the Building Blocks: Carbon, Hydrogen, and Oxygen
- Carbon (C): The Backbone of Life
- Hydrogen (H): A Versatile Partner
- Oxygen (O): The Reactive Element
- Functional Groups: The Key to Diversity
- 1. Hydroxyl Group (-OH): Alcohols and Sugars
- 2. Carbonyl Group (C=O): Aldehydes, Ketones, and Carboxylic Acids
- 3. Ether Group (C-O-C): Linking Molecules
- 4. Ester Group (RCOOR'): Fragrant Compounds
- Classes of CHO Compounds: A Diverse Family
- 1. Carbohydrates: The Body's Fuel
- 2. Alcohols: Versatile Solvents and Fuels
- 3. Carboxylic Acids: Acidic Components
- 4. Esters: Fragrant and Functional
- 5. Aldehydes and Ketones: Reactive Intermediates
- Applications of CHO Compounds: A Broad Spectrum
- 1. Medicine and Pharmaceuticals: Essential Components
- 2. Food and Beverages: Flavor and Energy
- 3. Cosmetics and Personal Care: Fragrances and Solvents
- 4. Industrial Processes: Solvents and Building Blocks
- 5. Biofuels: Renewable Energy Sources
- Analyzing CHO Compounds: Techniques and Methods
- 1. Spectroscopy: Unveiling Molecular Structure
- 2. Chromatography: Separating and Identifying Components
- 3. Elemental Analysis: Determining Composition
- The Future of CHO Compound Research: Ongoing Discoveries
- Latest Posts
- Latest Posts
- Related Post
A Compound Contains Only Carbon, Hydrogen, and Oxygen: Exploring the World of Organic Chemistry
Organic chemistry, the study of carbon-containing compounds, encompasses a vast and diverse landscape. A significant portion of this landscape is occupied by compounds containing only carbon, hydrogen, and oxygen (CHO). These compounds, ranging from simple sugars to complex polymers, play crucial roles in biological systems and numerous industrial applications. This article delves into the properties, characteristics, and diverse applications of compounds composed solely of carbon, hydrogen, and oxygen.
Understanding the Building Blocks: Carbon, Hydrogen, and Oxygen
Before exploring the myriad compounds formed from these three elements, let's briefly revisit their individual properties and how their combination leads to such a diverse array of molecules.
Carbon (C): The Backbone of Life
Carbon's unique ability to form four covalent bonds makes it the cornerstone of organic chemistry. This tetravalency allows carbon atoms to link together in chains, rings, and branched structures, creating the backbone of countless organic molecules. Its ability to form strong bonds with itself and other elements (hydrogen, oxygen, nitrogen, etc.) allows for the creation of incredibly complex and diverse structures.
Hydrogen (H): A Versatile Partner
Hydrogen, with its single electron, readily forms covalent bonds with carbon and oxygen. It's a crucial component in many organic compounds, contributing to their overall structure and properties. The presence and arrangement of hydrogen atoms significantly influence the reactivity and behavior of these molecules.
Oxygen (O): The Reactive Element
Oxygen, with its two unpaired electrons, is highly reactive and readily forms strong covalent bonds with both carbon and hydrogen. It often introduces functional groups into organic molecules, dramatically altering their properties and reactivity. The presence of oxygen atoms frequently determines the solubility, polarity, and biological activity of a CHO compound.
Functional Groups: The Key to Diversity
The arrangement of carbon, hydrogen, and oxygen atoms within a molecule, particularly the presence of specific functional groups, is the key determinant of its chemical and physical properties. These functional groups are specific groups of atoms within a molecule that are responsible for its characteristic chemical reactions. Several important functional groups found in CHO compounds include:
1. Hydroxyl Group (-OH): Alcohols and Sugars
The hydroxyl group is a defining feature of alcohols and sugars. Alcohols exhibit varying properties depending on the length and branching of the carbon chain to which the hydroxyl group is attached. Sugars, on the other hand, contain multiple hydroxyl groups and are vital energy sources in biological systems. The presence of hydroxyl groups enhances the solubility of these compounds in water.
2. Carbonyl Group (C=O): Aldehydes, Ketones, and Carboxylic Acids
The carbonyl group is a crucial functional group found in aldehydes, ketones, and carboxylic acids. Aldehydes have the carbonyl group at the end of a carbon chain, while ketones have it within the chain. Carboxylic acids possess a carbonyl group attached to a hydroxyl group, giving them acidic properties. These compounds exhibit diverse reactivity and find applications in various industries.
3. Ether Group (C-O-C): Linking Molecules
The ether group links two carbon atoms through an oxygen atom. Ethers are generally unreactive but serve as essential components in many organic solvents and biological molecules. They play a vital role in connecting different parts of larger molecules.
4. Ester Group (RCOOR'): Fragrant Compounds
Ester groups are formed by the reaction of a carboxylic acid with an alcohol. Esters are frequently responsible for the pleasant fragrances of fruits and flowers. Many esters are used in perfumes, flavorings, and other industries.
Classes of CHO Compounds: A Diverse Family
The combination of carbon, hydrogen, and oxygen, along with the presence of various functional groups, leads to several important classes of organic compounds:
1. Carbohydrates: The Body's Fuel
Carbohydrates are a crucial class of CHO compounds serving as primary energy sources for living organisms. They include simple sugars (monosaccharides like glucose and fructose), disaccharides (sucrose and lactose), and complex carbohydrates (polysaccharides like starch and cellulose). Their structure and properties determine their digestibility and metabolic functions.
2. Alcohols: Versatile Solvents and Fuels
Alcohols, characterized by the presence of a hydroxyl group (-OH), exhibit a wide range of properties and applications. Ethanol, a common alcohol, is used as a fuel, solvent, and in alcoholic beverages. Other alcohols find use in various industrial processes and as components of pharmaceuticals.
3. Carboxylic Acids: Acidic Components
Carboxylic acids, possessing a carboxyl group (-COOH), are organic acids with diverse applications. Acetic acid (vinegar) is a common example. Other carboxylic acids are used in the production of plastics, pharmaceuticals, and other materials.
4. Esters: Fragrant and Functional
Esters, formed from the reaction of carboxylic acids and alcohols, are known for their pleasant aromas. They are widely used in perfumes, flavorings, and as solvents. Some esters play important roles in biological processes.
5. Aldehydes and Ketones: Reactive Intermediates
Aldehydes and ketones, both containing the carbonyl group (C=O), are important intermediates in various chemical reactions. They are commonly used as solvents, reagents, and in the synthesis of other organic compounds. Formaldehyde, the simplest aldehyde, finds use as a preservative and disinfectant.
Applications of CHO Compounds: A Broad Spectrum
The applications of CHO compounds span across diverse fields:
1. Medicine and Pharmaceuticals: Essential Components
Many pharmaceuticals are CHO compounds or contain CHO components. Sugars are used in drug formulations. Many drugs contain alcohol or carboxylic acid functional groups.
2. Food and Beverages: Flavor and Energy
Carbohydrates are crucial energy sources in food. Esters provide characteristic flavors and aromas. Alcohols play a role in beverages.
3. Cosmetics and Personal Care: Fragrances and Solvents
Esters are widely used in perfumes and cosmetics. Alcohols serve as solvents in many personal care products.
4. Industrial Processes: Solvents and Building Blocks
Alcohols and esters are employed extensively as solvents in various industries. CHO compounds serve as building blocks for the synthesis of polymers and other materials.
5. Biofuels: Renewable Energy Sources
Ethanol, a CHO compound, is a prominent biofuel produced from renewable sources. Research is ongoing to explore other CHO compounds as potential biofuels.
Analyzing CHO Compounds: Techniques and Methods
Several techniques are employed to analyze CHO compounds and determine their structure and composition:
1. Spectroscopy: Unveiling Molecular Structure
Infrared (IR) spectroscopy provides information about the functional groups present in a molecule. Nuclear Magnetic Resonance (NMR) spectroscopy provides detailed information about the carbon and hydrogen atoms and their connectivity. Mass spectrometry (MS) determines the molecular weight and fragmentation pattern of the compound.
2. Chromatography: Separating and Identifying Components
Chromatographic techniques, such as gas chromatography (GC) and high-performance liquid chromatography (HPLC), are used to separate and identify individual components in a mixture of CHO compounds.
3. Elemental Analysis: Determining Composition
Elemental analysis provides the percentage of carbon, hydrogen, and oxygen in a CHO compound, which is crucial for determining its empirical formula.
The Future of CHO Compound Research: Ongoing Discoveries
Research on CHO compounds continues to expand, driven by the need for sustainable materials, renewable energy sources, and new pharmaceuticals. Areas of ongoing investigation include:
- Developing new biofuels: Research aims to identify and optimize CHO compounds for efficient and sustainable biofuel production.
- Designing new polymers: Scientists are exploring CHO-based polymers with enhanced properties for various applications.
- Synthesizing novel pharmaceuticals: Researchers are developing new CHO compounds with therapeutic potential.
- Understanding metabolic processes: Investigating the role of CHO compounds in biological systems to develop new therapies and improve human health.
In conclusion, compounds containing only carbon, hydrogen, and oxygen represent a vast and diverse class of organic molecules with critical roles in various aspects of life and industry. Their properties, determined by the arrangement of atoms and the presence of functional groups, dictate their applications. Ongoing research in this field promises new discoveries and innovations with significant impact on various sectors. Understanding these compounds is crucial for advancing our knowledge in chemistry, biology, medicine, and numerous other fields.
Latest Posts
Latest Posts
-
Select All The Characteristics Of Members Of Kingdom Fungi
Mar 26, 2025
-
For A Damped Oscillator With A Mass Of 200g
Mar 26, 2025
-
What Are The End Products Of Digestion Of Starch
Mar 26, 2025
-
The Middle Letter Of The Alphabet
Mar 26, 2025
-
What Is The Monomers Of Nucleic Acids
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about A Compound Contains Only Carbon Hydrogen And Oxygen . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
