A Cell Placed In A Hypertonic Solution Will
News Leon
Mar 15, 2025 · 5 min read
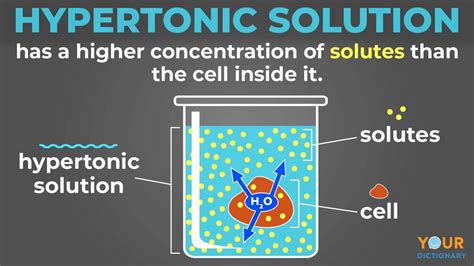
Table of Contents
A Cell Placed in a Hypertonic Solution Will: Understanding Osmosis and its Effects
Osmosis, the passive movement of water across a selectively permeable membrane, is a fundamental process in biology with significant implications for cell survival and function. Understanding what happens when a cell is placed in a hypertonic solution is crucial for comprehending cellular processes, from maintaining hydration in plants to understanding the effects of dehydration in animals. This article delves deep into the mechanics of osmosis, specifically focusing on the consequences for a cell immersed in a hypertonic environment.
Understanding Osmosis: The Basics
Before we explore the effects on a cell, let's solidify our understanding of osmosis. Osmosis is driven by the difference in water potential between two solutions separated by a semipermeable membrane. Water potential is the tendency of water to move from one area to another; it's influenced by factors like solute concentration and pressure.
- Hypotonic Solution: A solution with a lower solute concentration (and therefore higher water potential) compared to the inside of a cell.
- Hypertonic Solution: A solution with a higher solute concentration (and therefore lower water potential) compared to the inside of a cell.
- Isotonic Solution: A solution with an equal solute concentration compared to the inside of a cell.
Water naturally moves from an area of high water potential (hypotonic) to an area of low water potential (hypertonic) across the selectively permeable membrane until equilibrium is reached. This membrane allows water molecules to pass through but restricts the movement of larger solute molecules.
What Happens When a Cell is Placed in a Hypertonic Solution?
When a cell is placed in a hypertonic solution, the water potential inside the cell is higher than in the surrounding solution. This creates a concentration gradient, driving water to move out of the cell and into the hypertonic solution. This outward movement of water leads to several significant consequences, depending on the type of cell:
Effects on Animal Cells
Animal cells, lacking a rigid cell wall, are particularly vulnerable to hypertonic environments. As water leaves the cell, the cell shrinks and undergoes a process called crenation. This shrinkage can disrupt cellular processes, affecting the cell's ability to function properly. Severe crenation can lead to cell death. The loss of water significantly reduces cell volume and can damage cellular components. This process is often observed in red blood cells when placed in a concentrated salt solution.
Effects on Plant Cells
Plant cells, possessing a rigid cell wall, respond differently to hypertonic solutions. While water still moves out of the cell, the cell wall prevents the cell from completely collapsing. Instead, the cell membrane pulls away from the cell wall, a process known as plasmolysis. This separation creates gaps between the cell membrane and wall. While plasmolysis doesn't necessarily lead to immediate cell death, it significantly impairs the cell's function. The loss of turgor pressure, the pressure exerted by the cell contents against the cell wall, causes the plant to wilt. This wilting is a visible manifestation of plasmolysis.
Turgor pressure, crucial for maintaining plant structure and facilitating nutrient transport, is greatly reduced during plasmolysis. The process is reversible if the plant is rehydrated, allowing the cell to regain turgor and its normal shape. However, prolonged plasmolysis can lead to irreversible damage and ultimately cell death.
Mechanisms and Factors Influencing Osmosis
Several factors influence the rate and extent of osmosis in a hypertonic environment:
Permeability of the Cell Membrane
The cell membrane's permeability is a critical factor. A more permeable membrane will allow for a faster rate of water movement. The presence of aquaporins, specialized protein channels that facilitate water transport, can significantly increase the rate of osmosis.
Solute Concentration Gradient
The difference in solute concentration between the cell and the surrounding solution directly determines the driving force for osmosis. A steeper concentration gradient (a greater difference in solute concentration) leads to faster water movement out of the cell.
Surface Area to Volume Ratio
The surface area to volume ratio of the cell influences the rate of water loss. Cells with a higher surface area to volume ratio will experience a faster rate of water loss due to increased surface area available for water movement.
Temperature
Temperature affects the kinetic energy of water molecules. Higher temperatures lead to faster water movement, accelerating the rate of osmosis.
Real-World Examples and Applications
The effects of hypertonic solutions are observable in numerous real-world situations:
Food Preservation
Hypertonic solutions are used in food preservation techniques such as salting and sugaring. The high solute concentration in salt or sugar solutions draws water out of microorganisms, inhibiting their growth and preventing food spoilage.
Medical Applications
Understanding osmosis is critical in various medical applications. Intravenous (IV) solutions are carefully formulated to be isotonic to prevent damage to red blood cells. Dehydration treatment often involves administering isotonic fluids to restore proper fluid balance.
Plant Physiology
Understanding plasmolysis is essential in plant physiology. Farmers and gardeners use osmotic principles to adjust watering practices and soil salinity to ensure optimal plant health. The wilting of plants during drought is a direct consequence of water loss due to a hypertonic soil solution.
Marine Biology
Marine organisms are constantly adapting to osmotic challenges. Marine fish live in a hypertonic environment and have evolved mechanisms to regulate water balance and prevent dehydration. Their kidneys are highly efficient at removing excess salt from their bodies, a crucial adaptation for survival.
Conclusion
Placing a cell in a hypertonic solution results in water moving out of the cell, leading to crenation in animal cells and plasmolysis in plant cells. These processes significantly impact cell function and survival, highlighting the importance of maintaining osmotic balance. Understanding the principles of osmosis and its effects is crucial in various scientific disciplines, from medicine and agriculture to marine biology and environmental science. The consequences of hypertonic environments underscore the delicate balance required for cellular health and overall organism survival. The reversible nature of plasmolysis in plants provides a mechanism for adaptation to fluctuating water availability, while the irreversible damage caused by crenation in animal cells highlights the vulnerability of cells to drastic osmotic imbalances. Continued research into the cellular responses to hypertonic stress continues to illuminate intricate cellular mechanisms and opens avenues for developing strategies to mitigate the damaging effects of osmotic imbalances.
Latest Posts
Latest Posts
-
If A Pea Plant Shows A Recessive Phenotype
Mar 15, 2025
-
Is A Patent A Current Asset
Mar 15, 2025
-
Why Are Producers So Important To An Ecosystem
Mar 15, 2025
-
Difference Between Interest Groups And Political Parties
Mar 15, 2025
-
The Division Of The Cell Nucleus Is Called
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about A Cell Placed In A Hypertonic Solution Will . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
