A Catalyst Speeds Up A Chemical Reaction By
News Leon
Mar 14, 2025 · 6 min read
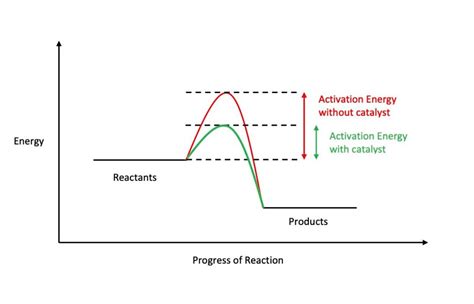
Table of Contents
A Catalyst Speeds Up a Chemical Reaction By: Lowering Activation Energy
Chemical reactions are the foundation of all processes occurring in the world around us. From the rusting of iron to the complex metabolic pathways within our bodies, these reactions dictate the pace of change. However, many reactions proceed at a painfully slow rate, hindering their practical applications. This is where catalysts enter the scene, dramatically accelerating the speed of these reactions without being consumed themselves. But how exactly do they achieve this remarkable feat? The answer lies in their ability to lower the activation energy of a reaction.
Understanding Activation Energy: The Energy Barrier
Before we delve into the role of catalysts, it’s crucial to grasp the concept of activation energy (Ea). Imagine a chemical reaction as a ball rolling down a hill. The hill represents the energy barrier that reactants must overcome to transform into products. The height of this hill is the activation energy – the minimum energy required for the reactants to reach the transition state, an unstable intermediate state before forming products.
A reaction with a high activation energy proceeds slowly because only a small fraction of reactant molecules possess enough kinetic energy to surmount this energy barrier at a given temperature. Conversely, reactions with low activation energies are faster, as a larger proportion of molecules possess the necessary energy.
The Role of Collisions in Chemical Reactions
Chemical reactions happen when reactant molecules collide with sufficient energy and proper orientation. The collision must be energetic enough to break existing bonds and create new ones, leading to the formation of products. The activation energy dictates the minimum energy required for a successful, product-forming collision.
Catalysts: The Reaction Accelerators
Catalysts are substances that significantly increase the rate of a chemical reaction without being consumed in the process. They achieve this acceleration by providing an alternative reaction pathway with a lower activation energy. Think of it as building a tunnel through the hill, providing a shorter, easier route for the ball to roll down.
How Catalysts Lower Activation Energy
Catalysts lower the activation energy through several mechanisms:
-
Providing an alternative reaction pathway: Catalysts offer a different route for the reaction to proceed, one with a lower energy barrier. This new pathway might involve the catalyst forming intermediate complexes with reactants, stabilizing the transition state, or facilitating the breaking and forming of bonds in a more efficient way.
-
Stabilizing the transition state: The transition state is a high-energy, unstable intermediate formed during the reaction. Catalysts can bind to the reactants, forming a catalyst-reactant complex that resembles the transition state but is lower in energy. This stabilization lowers the overall activation energy.
-
Orienting reactants: Reactants must collide with the correct orientation for a reaction to occur. Catalysts can bring reactants together in the proper orientation, increasing the likelihood of a successful collision. This orientation effect contributes to a higher reaction rate.
-
Surface area: Heterogeneous catalysts, which are in a different phase than the reactants (e.g., a solid catalyst in a liquid reaction), provide a large surface area for the reaction to occur. This increased surface area increases the number of reactant molecules that can interact with the catalyst simultaneously.
Types of Catalysis
Catalysis can be broadly categorized into two main types:
1. Homogeneous Catalysis
In homogeneous catalysis, the catalyst and reactants are in the same phase (e.g., all are in the liquid phase). The catalyst participates directly in the reaction mechanism, forming intermediate complexes with the reactants. Once the reaction is complete, the catalyst is regenerated in its original form.
Example: The use of a strong acid (like sulfuric acid) to catalyze the esterification reaction between a carboxylic acid and an alcohol. The acid protonates the carboxylic acid, making it more reactive toward the alcohol.
2. Heterogeneous Catalysis
In heterogeneous catalysis, the catalyst and reactants are in different phases. A common example is a solid catalyst used in a gaseous or liquid reaction. The reaction occurs at the surface of the catalyst. The reactants adsorb onto the catalyst surface, undergo reaction, and then the products desorb from the surface.
Example: The Haber-Bosch process for ammonia synthesis uses an iron catalyst. Nitrogen and hydrogen gases adsorb onto the iron surface, where they react to form ammonia, which then desorbs.
Examples of Catalysts in Everyday Life and Industry
Catalysts play a crucial role in numerous industrial processes and natural phenomena. Here are a few notable examples:
-
Automotive catalytic converters: These devices use platinum, palladium, and rhodium catalysts to convert harmful exhaust gases (like carbon monoxide, nitrogen oxides, and unburned hydrocarbons) into less harmful substances (carbon dioxide, nitrogen, and water).
-
Enzymes: Enzymes are biological catalysts that accelerate countless reactions within living organisms. They are highly specific, catalyzing only particular reactions. Examples include digestive enzymes that break down food and enzymes involved in DNA replication.
-
Polymerization catalysts: These catalysts are essential for the production of plastics and other polymers. They facilitate the chain growth reaction by which monomers join together to form long polymer chains.
-
Petroleum refining: Catalysts are widely used in petroleum refining to convert crude oil into various fuels and petrochemicals. These catalysts facilitate cracking, reforming, and isomerization reactions, optimizing the properties of the resulting products.
Factors Affecting Catalytic Activity
Several factors influence the activity and effectiveness of catalysts:
-
Temperature: Increasing temperature generally increases the rate of a catalyzed reaction, but excessively high temperatures can damage or deactivate some catalysts.
-
Pressure: In gas-phase reactions, increasing pressure can enhance the rate of reaction by increasing the concentration of reactants at the catalyst surface.
-
Catalyst concentration: Increasing the catalyst concentration (for homogeneous catalysis) or surface area (for heterogeneous catalysis) generally increases the reaction rate, up to a point where saturation occurs.
-
Presence of inhibitors or poisons: Certain substances can bind to the active sites of a catalyst, blocking its activity. These substances are called inhibitors or catalyst poisons.
Conclusion: The Unsung Heroes of Chemistry
Catalysts are indispensable in countless aspects of our lives. Their ability to dramatically accelerate chemical reactions underpins crucial industrial processes, enables the production of essential materials, and is fundamental to the functioning of living organisms. By lowering the activation energy of chemical reactions, catalysts unlock the potential of numerous transformations that would otherwise be prohibitively slow or impractical. Understanding the mechanisms by which catalysts operate provides valuable insights into reaction kinetics and opens avenues for the development of more efficient and sustainable catalytic processes. The ongoing research and innovation in catalysis continue to push the boundaries of chemical possibilities, promising further advancements in various fields of science and technology. From developing cleaner energy sources to designing more efficient manufacturing processes, catalysts are the unsung heroes of chemical transformations, driving progress and shaping our future.
Latest Posts
Latest Posts
-
Transactions Are Recorded In A Journal In
Mar 14, 2025
-
Which Of The Following Needs A Proof
Mar 14, 2025
-
Pure Substances Are Made Of Only One Type Of
Mar 14, 2025
-
A Dpt Vaccination Is An Example Of
Mar 14, 2025
-
Whats The Difference Between An Enzyme And A Hormone
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about A Catalyst Speeds Up A Chemical Reaction By . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
