10 Protons 11 Neutrons 10 Electrons
News Leon
Apr 06, 2025 · 5 min read
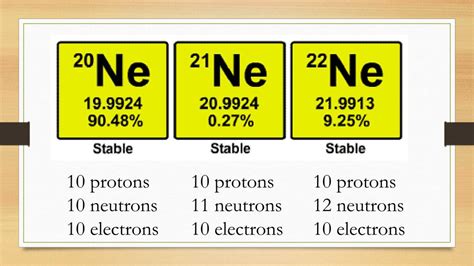
Table of Contents
10 Protons, 11 Neutrons, 10 Electrons: Unveiling the Mystery of Isotopes and Ions
The seemingly simple combination of 10 protons, 11 neutrons, and 10 electrons might seem insignificant at first glance. However, this specific arrangement delves into the fascinating world of atomic structure, isotopes, and ions – fundamental concepts in chemistry and physics. Understanding this configuration unlocks a deeper appreciation of how matter behaves at its most basic level. This article will explore the implications of this unique atomic composition, explaining the concepts involved and their broader significance in science.
Understanding the Basics: Protons, Neutrons, and Electrons
Before diving into the specifics of our 10-11-10 configuration, let's establish a clear understanding of the fundamental subatomic particles:
-
Protons: Positively charged particles residing in the atom's nucleus. The number of protons defines the element; an atom with 10 protons is always Neon (Ne).
-
Neutrons: Neutral particles (no charge) also found in the nucleus. Neutrons contribute to the atom's mass but not its charge.
-
Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. Electrons determine an atom's chemical properties and its ability to form bonds with other atoms. The number of electrons typically equals the number of protons in a neutral atom.
The Significance of 10 Protons: Neon
The presence of 10 protons unequivocally identifies the element as Neon (Ne). Neon is a noble gas, meaning it's chemically inert; it rarely forms chemical bonds with other elements due to its stable electron configuration (a full outermost electron shell). This inertness contributes to its unique properties and applications, most notably in lighting and signage. Neon's stability is a direct consequence of its electronic structure, dictated by the 10 protons in its nucleus.
The Role of 11 Neutrons: Isotopes and Atomic Mass
The 11 neutrons are where things get interesting. The number of neutrons in an atom's nucleus can vary, even while the number of protons remains constant. Atoms of the same element with differing numbers of neutrons are called isotopes. Neon has several naturally occurring isotopes, each with a different number of neutrons.
Our 10-11-10 configuration represents an isotope of Neon. To be precise, we need to identify the mass number. The mass number is the sum of protons and neutrons (10 + 11 = 21). This specific isotope is denoted as Neon-21 (²¹Ne). Other Neon isotopes exist, such as Neon-20 (²⁰Ne) and Neon-22 (²²Ne), each with a slightly different abundance in nature. The varying number of neutrons affects the isotope's mass and, to a lesser extent, its stability. Some isotopes are stable, while others are radioactive, undergoing decay to achieve a more stable configuration.
The Impact of 10 Electrons: Ions and Charge
The presence of only 10 electrons, while there are 10 protons, is crucial. In a neutral atom, the number of protons and electrons are equal, resulting in a net charge of zero. However, in our 10-11-10 configuration, we have a situation where the number of electrons is equal to the number of protons. This means that the atom is electrically neutral. It is a neutral atom of the Neon-21 isotope.
Neon-21: Properties and Applications
Neon-21, like other Neon isotopes, is a noble gas. Its chemical inertness limits its direct applications compared to reactive elements. However, its properties are exploited in specialized fields:
1. Scientific Research:
-
Nuclear Physics: Neon isotopes are used in nuclear physics research to study nuclear reactions and properties. The slightly different masses of different Neon isotopes allows scientists to observe their behavior in various experiments.
-
Mass Spectrometry: Different Neon isotopes have slightly different mass-to-charge ratios. This property makes them useful in mass spectrometry, a technique used to identify and quantify the different components in a sample.
2. Medical Applications:
- Medical Imaging: While not directly using Neon-21 specifically, Neon isotopes might find application in certain medical imaging techniques. The use of noble gases in imaging is rare, but research into their properties continues.
3. Industrial Applications:
- Laser Technology: Though not directly using Neon-21, other Neon isotopes are sometimes used in specific laser types. This is due to Neon's unique spectral properties.
Contrasting with Ions: A Different Scenario
It's important to contrast our neutral Neon-21 atom with Neon ions. If our 10-11-10 configuration had an unequal number of protons and electrons (e.g., 10 protons, 11 neutrons, and 9 electrons), we'd have a Neon ion, specifically a Neon cation (Ne⁺) because it would carry a positive charge due to having one more proton than electrons. Similarly, if it had 11 electrons, it would be a Neon anion (Ne⁻) with a negative charge. Ions exhibit vastly different chemical properties compared to neutral atoms. They readily participate in chemical reactions and form ionic bonds.
The Broader Context: Isotopes and Their Significance
The concept of isotopes extends far beyond Neon. Many elements possess multiple isotopes, some stable and others radioactive. Radioactive isotopes have crucial applications in various fields:
-
Medicine: Radioactive isotopes are used in medical imaging (PET scans) and cancer therapy.
-
Archaeology: Radiocarbon dating, utilizing the radioactive isotope Carbon-14, helps determine the age of ancient artifacts.
-
Industrial Applications: Radioactive isotopes are used in various industrial processes, such as gauging the thickness of materials and tracing the flow of liquids.
Conclusion: The Significance of Atomic Composition
The seemingly simple combination of 10 protons, 11 neutrons, and 10 electrons is more than just a numerical arrangement. It reveals the core principles of atomic structure, isotopes, and ions. Understanding these concepts is crucial for comprehending the properties of matter and their applications in science and technology. The study of isotopes, in particular, has revolutionized various fields, ranging from medicine and archaeology to industrial processes and fundamental scientific research. The unique properties of Neon-21, while not as widely exploited as some other isotopes, highlight the importance of investigating the subtle differences in atomic composition and their impact on the overall behavior of matter. Further research into the specific applications and properties of Neon-21 and other less-studied isotopes could uncover even more fascinating possibilities in the future.
Latest Posts
Latest Posts
-
The Ph Of 0 1 Molar Ammonia Is Approximately
Apr 08, 2025
-
Is Na A Metal Nonmetal Or Metalloid
Apr 08, 2025
-
What Subatomic Particle Is The Heaviest
Apr 08, 2025
-
4 Is What Percent Of 200
Apr 08, 2025
-
What Is The Oxidation State Of Carbon In Glucose C6h12o6
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about 10 Protons 11 Neutrons 10 Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.